Structure of Benzene, Orbital Picture, Resonance in Benzene, Aromatic Characters, Huckel’S Rule B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heterocyclic Chemistry Updated
1 | Page Heterocyclic Chemistry By Dr Vipul Kataria Definition: A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements (N, O, S, P) as members of its ring. Introduction: Heterocyclic compounds may be defined as cyclic compounds having as ring members atoms of at least two different elements. It means the cyclic compound has one another atom different than carbon. Heterocyclic compounds have much importance in organic chemistry because of abundant presence of heterocyclic compounds in present pharmaceutical drugs. Like other organic compounds, there are several defined rules for naming heterocyclic compounds. IUPAC rules for nomenclature of heterocyclic systems (SPU 2012, 2 Marks) The system for nomenclature for heterocyclic systems was given by Hantzsch and Widman. The Hantzsch-Widman nomenclature is based on the type (Z) of the heteroatom; the ring size (n) and nature of the ring, whether it is saturated or unsaturated. This system of nomenclature applies to monocyclic 3-to-10-membered ring heterocycles. Type of the heteroatom: The type of heteroatom is indicated by a prefix as shown below for common hetreroatoms. Dr Vipul Kataria, Chemistry Department, V. P. & R. P. T. P. Science College, VV Nagar 2 | Pag e When two or more of the same heteroatoms are present, the prrefix di, tri, tetra… are used. e.g. dioxa, triaza, dithia. If the heteroatoms are different their atomic number in that grroup is considered. Thus order of numbering will be O, S, N, P, Si. Ring size: The ring size is indicated by a suffix according to Table I below. -

Condensed Cyclic Compound, Composition Including The
(19) TZZ¥_ _T (11) EP 3 184 522 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 28.06.2017 Bulletin 2017/26 C07D 403/14 (2006.01) H01L 51/00 (2006.01) (21) Application number: 16205894.5 (22) Date of filing: 21.12.2016 (84) Designated Contracting States: • INAYAMA, Satoshi AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Yokohama-city, Kanagawa 230-0027 (JP) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • ISHII, Norihito PL PT RO RS SE SI SK SM TR Yokohama-city, Kanagawa 230-0027 (JP) Designated Extension States: • SHIBATA, Katsunori BA ME Yokohama-city, Kanagawa 230-0027 (JP) Designated Validation States: • ITO, Mitsunori MA MD Yokohama-city, Kanagawa 230-0027 (JP) (30) Priority: 22.12.2015 JP 2015250531 (74) Representative: Scheuermann, Erik Elkington and Fife LLP (71) Applicant: Samsung Electronics Co., Ltd. Prospect House Gyeonggi-do 16677 (KR) 8 Pembroke Road Sevenoaks, Kent TN13 1XR (GB) (72) Inventors: • KATO, Fumiaki Yokohama-city, Kanagawa 230-0027 (JP) (54) CONDENSED CYCLIC COMPOUND, COMPOSITION INCLUDING THE CONDENSED CYCLIC COMPOUND, ORGANIC LIGHT-EMITTING DEVICE INCLUDING THE CONDENSED CYCLIC COMPOUND, AND METHOD OF MANUFACTURING THE ORGANIC LIGHT-EMITTING DEVICE (57) A condensed cyclic compound, a composition including the condensed cyclic compound, an organic light-emit- ting device including the condensed cyclic compound, and a method of manufacturing the organic light-emitting device are provided. EP 3 184 522 A1 Printed by Jouve, 75001 PARIS (FR) EP 3 184 522 A1 Description FIELD OF THE INVENTION 5 [0001] One or more embodiments relate to a condensed cyclic compound, a composition including the condensed cyclic compound, an organic light-emitting device including the condensed cyclic compound, and a method of manufac- turing the organic light-emitting device. -

Polar Covalent Bonds: Electronegativity
Polar Covalent Bonds: Electronegativity Covalent bonds can have ionic character These are polar covalent bonds Bonding electrons attracted more strongly by one atom than by the other Electron distribution between atoms is not symmetrical Bond Polarity and Electronegativity Symmetrical Covalent Bonds Polar Covalent Bonds C – C + - C – H C – O (non-polar) (polar) Electronegativity (EN): intrinsic ability of an atom to attract the shared electrons in a covalent bond Inductive Effect: shifting of sigma bonded electrons in resppygonse to nearby electronegative atom The Periodic Table and Electronegativity C – H C - Br and C - I (non-pol)lar) (po l)lar) Bond Polarity and Inductive Effect Nonpolar Covalent Bonds: atoms with similar EN Polar Covalent Bonds: Difference in EN of atoms < 2 Ionic Bonds: Difference in EN > 2 C–H bonds, relatively nonpolar C-O, C-X bonds (more electronegative elements) are polar Bonding electrons shift toward electronegative atom C acqqppuires partial positive char g,ge, + Electronegative atom acquires partial negative charge, - Inductive effect: shifting of electrons in a bond in response to EN of nearby atoms Electrostatic Potential Maps Electrostatic potential maps show calculated charge distributions Colors indicate electron- rich (red) and electron- poor (blue ) reg ions Arrows indicate direction of bond polarity Polar Covalent Bonds: Net Dipole Moments Molecules as a whole are often polar from vector summation of individual bond polarities and lone-pair contributions Strongly polar substances soluble in polar solvents like water; nonpolar substances are insoluble in water. Dipole moment ( ) - Net molecular polarity, due to difference in summed charges - magnitude of charge Q at end of molecular dipole times distance r between charges = Q r, in debyes (D), 1 D = 3.336 1030 coulomb meter length of an average covalent bond, the dipole moment would be 1.60 1029 Cm, or 4.80 D. -

Organic Chemistry PEIMS Code: N1120027 Abbreviation: ORGCHEM Number of Credits That May Be Earned: 1/2-1
Course: Organic Chemistry PEIMS Code: N1120027 Abbreviation: ORGCHEM Number of credits that may be earned: 1/2-1 Brief description of the course (150 words or less): Organic chemistry is an introductory course that is designed for the student who intends to continue future study in the sciences. The student will learn the concepts and applications of organic chemistry. Topics covered include aliphatic and aromatic compounds, alcohols, aldehydes, ketones, acids, ethers, amines, spectra, and stereochemistry. A brief introduction into biochemistry is also provided. The laboratory experiments will familiarize the student with the important laboratory techniques, specifically spectroscopy. Traditional high school chemistry courses focus on the inorganic aspects of chemistry whereas organic chemistry introduces the student to organic compounds and their properties, mechanisms of formations, and introduces the student to laboratory techniques beyond the traditional high school chemistry curriculum. Essential Knowledge and Skills of the course: (a) General requirements. This course is recommended for students in Grades 11-12. The recommended prerequisite for this course is AP Chemistry. (b) Introduction. Organic chemistry is designed to introduce students to the fundamental concepts of organic chemistry and key experimental evidence and data, which support these concepts. Students will learn to apply these data and concepts to chemical problem solving. Additionally, students will learn that organic chemistry is still evolving by reading about current breakthroughs in the field. Finally, students will gain appreciation for role that organic chemistry plays in modern technological developments in diverse fields, ranging from biology to materials science. (c) Knowledge and skills. (1) The student will be able to write both common an IUPAC names for the hydrocarbon. -

Heterocycles 2 Daniel Palleros
Heterocycles 2 Daniel Palleros Heterocycles 1. Structures 2. Aromaticity and Basicity 2.1 Pyrrole 2.2 Imidazole 2.3 Pyridine 2.4 Pyrimidine 2.5 Purine 3. Π-excessive and Π-deficient Heterocycles 4. Electrophilic Aromatic Substitution 5. Oxidation-Reduction 6. DNA and RNA Bases 7. Tautomers 8. H-bond Formation 9. Absorption of UV Radiation 10. Reactions and Mutations Heterocycles 3 Daniel Palleros Heterocycles Heterocycles are cyclic compounds in which one or more atoms of the ring are heteroatoms: O, N, S, P, etc. They are present in many biologically important molecules such as amino acids, nucleic acids and hormones. They are also indispensable components of pharmaceuticals and therapeutic drugs. Caffeine, sildenafil (the active ingredient in Viagra), acyclovir (an antiviral agent), clopidogrel (an antiplatelet agent) and nicotine, they all have heterocyclic systems. O CH3 N HN O O N O CH 3 N H3C N N HN N OH O S O H N N N 2 N O N N O CH3 N CH3 caffeine sildenafil acyclovir Cl S N CH3 N N H COOCH3 nicotine (S)-clopidogrel Here we will discuss the chemistry of this important group of compounds beginning with the simplest rings and continuing to more complex systems such as those present in nucleic acids. Heterocycles 4 Daniel Palleros 1. Structures Some of the most important heterocycles are shown below. Note that they have five or six-membered rings such as pyrrole and pyridine or polycyclic ring systems such as quinoline and purine. Imidazole, pyrimidine and purine play a very important role in the chemistry of nucleic acids and are highlighted. -

8.3 Bonding Theories >
8.3 Bonding Theories > Chapter 8 Covalent Bonding 8.1 Molecular Compounds 8.2 The Nature of Covalent Bonding 8.3 Bonding Theories 8.4 Polar Bonds and Molecules 1 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals Molecular Orbitals How are atomic and molecular orbitals related? 2 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals • The model you have been using for covalent bonding assumes the orbitals are those of the individual atoms. • There is a quantum mechanical model of bonding, however, that describes the electrons in molecules using orbitals that exist only for groupings of atoms. 3 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals • When two atoms combine, this model assumes that their atomic orbitals overlap to produce molecular orbitals, or orbitals that apply to the entire molecule. 4 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals Just as an atomic orbital belongs to a particular atom, a molecular orbital belongs to a molecule as a whole. • A molecular orbital that can be occupied by two electrons of a covalent bond is called a bonding orbital. 5 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals Sigma Bonds When two atomic orbitals combine to form a molecular orbital that is symmetrical around the axis connecting two atomic nuclei, a sigma bond is formed. • Its symbol is the Greek letter sigma (σ). -

Molecular Orbital Theory
Molecular Orbital Theory The Lewis Structure approach provides an extremely simple method for determining the electronic structure of many molecules. It is a bit simplistic, however, and does have trouble predicting structures for a few molecules. Nevertheless, it gives a reasonable structure for many molecules and its simplicity to use makes it a very useful tool for chemists. A more general, but slightly more complicated approach is the Molecular Orbital Theory. This theory builds on the electron wave functions of Quantum Mechanics to describe chemical bonding. To understand MO Theory let's first review constructive and destructive interference of standing waves starting with the full constructive and destructive interference that occurs when standing waves overlap completely. When standing waves only partially overlap we get partial constructive and destructive interference. To see how we use these concepts in Molecular Orbital Theory, let's start with H2, the simplest of all molecules. The 1s orbitals of the H-atom are standing waves of the electron wavefunction. In Molecular Orbital Theory we view the bonding of the two H-atoms as partial constructive interference between standing wavefunctions of the 1s orbitals. The energy of the H2 molecule with the two electrons in the bonding orbital is lower by 435 kJ/mole than the combined energy of the two separate H-atoms. On the other hand, the energy of the H2 molecule with two electrons in the antibonding orbital is higher than two separate H-atoms. To show the relative energies we use diagrams like this: In the H2 molecule, the bonding and anti-bonding orbitals are called sigma orbitals (σ). -

AROMATIC COMPOUNDS Aromaticity
AROMATICITY AROMATIC COMPOUNDS Aromaticity Benzene - C6H6 H H H H H H H H H H H H Kekulé and the Structure of Benzene Kekule benzene: two forms are in rapid equilibrium 154 pm 134 pm • All bonds are 140 pm (intermediate between C-C and C=C) • C–C–C bond angles are 120° • Structure is planar, hexagonal A Resonance Picture of Bonding in Benzene resonance hybrid 6 -electron delocalized over 6 carbon atoms The Stability of Benzene Aromaticity: cyclic conjugated organic compounds such as benzene, exhibit special stability due to resonance delocalization of -electrons. Heats of hydrogenation + H2 + 120 KJ/mol + 2 H2 + 230 KJ/mol calc'd value= 240 KJ/mol 10 KJ/mol added stability + 3 H 2 + 208 KJ/mol calc'd value= 360 KJ/mol 152 KJ/mol added stability + 3 H2 + 337 KJ/mol 1,3,5-Hexatriene - conjugated but not cyclic Resonance energy of benzene is 129 - 152 KJ/mol An Orbital Hybridization View of Bonding in Benzene • Benzene is a planar, hexagonal cyclic hydrocarbon • The C–C–C bond angles are 120° = sp2 hybridized • Each carbon possesses an unhybridized p-orbital, which makes up the conjugated -system. • The six -electrons are delocalized through the -system The Molecular Orbitals of Benzene - the aromatic system of benzene consists of six p-orbitals (atomic orbitals). Benzene must have six molecular orbitals. Y6 Y4 Y5 six p-orbitals Y2 Y3 Y1 Degenerate orbitals: Y : zero nodes orbitals that have the 1 Bonding same energy Y2 and Y3: one node Y and Y : two nodes 4 5 Anti-bonding Y6: three node Substituted Derivatives of Benzene and Their Nomenclature -
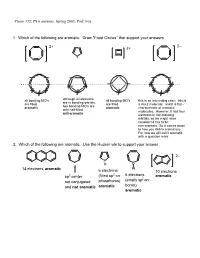
PS 6 Answers, Spring 2003, Prof
Chem 332, PS 6 answers, Spring 2003, Prof. Fox 1. Which of the following are aromatic. Draw "Frost Circles" that support your answers 2– 2+ 2+ + all bonding MO's although all electrons all bonding MO's this is an interesting case: this is are in bonding orbitals, are filled are filled a 4n+2 molecule, and it is flat-- two bonding MO's are aromatic aromatic characteristic of aromatic only half-filled molecules. However, it has four anti-aromatic electrons in non-bonding orbitals, so we might have considered this to be non-aromatic. So it comes down to how you define aromaticity. For now we will call it aromatic with a question mark. 2. Which of the following are aromatic. Use the Huckel rule to support your answer 2– P H B 14 electrons, aromatic H 6 electrons 10 electrons 2 6 electrons sp3 center (filled sp on aromatic (empty sp2 on not conjugated phosphorus) boron) and not aromatic aromatic aromatic 3. Compound 1 has an unusually large dipole moment for an organic hydrocarbon. Explain why (consider resonance structures for the central double bond) We need to remember that for any double bond compound, we can draw two polar resonance structure. Normally, these resonance structures are unimportant unimportant unimportant However, for 1 the story is different because there is a polar resonance form that has two aromatic components. In other words, 'breaking' the double bond gives rise to aromaticity, and therefore the polar resonance structure is important and leads to the observed dipole. dipole 1 8 e– 4 e– 6 e– 6 e– anti- anti- aromatic aromatic aromatic aromatic important resonance unimportant resonance structure structure 5. -

Inorganic Chemistry/Chemical Bonding/MO Diagram 1
Inorganic Chemistry/Chemical Bonding/MO Diagram 1 Inorganic Chemistry/Chemical Bonding/MO Diagram A molecular orbital diagram or MO diagram for short is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the Linear combination of atomic orbitals molecular orbital method (LCAO method) in particular [1] [2] [3] . This tool is very well suited for simple diatomic molecules such as dihydrogen, dioxygen and carbon monoxide but becomes more complex when discussing polynuclear molecules such as methane. It explains why some molecules exist and not others, how strong bonds are, and what electronic transitions take place. Dihydrogen MO diagram The smallest molecule, hydrogen gas exists as dihydrogen (H-H) with a single covalent bond between two hydrogen atoms. As each hydrogen atom has a single 1s atomic orbital for its electron, the bond forms by overlap of these two atomic orbitals. In figure 1 the two atomic orbitals are depicted on the left and on the right. The vertical axis always represents the orbital energies. The atomic orbital energy correlates with electronegativity as a more electronegative atom holds an electron more tightly thus lowering its energy. MO treatment is only valid when the atomic orbitals have comparable energy; when they differ greatly the mode of bonding becomes ionic. Each orbital is singly occupied with the up and down arrows representing an electron. The two AO's can overlap in two ways depending on their phase relationship. The phase of an orbital is a direct consequence of the oscillating, wave-like properties of electrons. -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -

Biomoleculesbiomolecules
1414Unit Objectives BiomoleculesBiomolecules After studying this Unit, you will be able to • explain the characteristics of “It is the harmonious and synchronous progress of chemical biomolecules like carbohydrates, reactions in body which leads to life”. proteins and nucleic acids and hormones; • classify carbohydrates, proteins, A living system grows, sustains and reproduces itself. nucleic acids and vitamins on the The most amazing thing about a living system is that it basis of their structures; is composed of non-living atoms and molecules. The • explain the difference between pursuit of knowledge of what goes on chemically within DNA and RNA; a living system falls in the domain of biochemistry. Living • describe the role of biomolecules systems are made up of various complex biomolecules in biosystem. like carbohydrates, proteins, nucleic acids, lipids, etc. Proteins and carbohydrates are essential constituents of our food. These biomolecules interact with each other and constitute the molecular logic of life processes. In addition, some simple molecules like vitamins and mineral salts also play an important role in the functions of organisms. Structures and functions of some of these biomolecules are discussed in this Unit. 14.114.114.1 Carbohydrates Carbohydrates are primarily produced by plants and form a very large group of naturally occurring organic compounds. Some common examples of carbohydrates are cane sugar, glucose, starch, etc. Most of them have a general formula, Cx(H2O)y, and were considered as hydrates of carbon from where the name carbohydrate was derived. For example, the molecular formula of glucose (C6H12O6) fits into this general formula, C6(H2O)6. But all the compounds which fit into this formula may not be classified as carbohydrates.