Physics of Resonating Valence Bond Spin Liquids Julia Saskia Wildeboer Washington University in St
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Arxiv:2007.11161V3 [Cond-Mat.Str-El] 13 Mar 2021](https://docslib.b-cdn.net/cover/7621/arxiv-2007-11161v3-cond-mat-str-el-13-mar-2021-227621.webp)
Arxiv:2007.11161V3 [Cond-Mat.Str-El] 13 Mar 2021
Topological phase transition and single/multi anyon dynamics of /2 spin liquid Zheng Yan,1 Yan-Cheng Wang,2 Nvsen Ma,3 Yang Qi,4, 5, 6, ∗ and Zi Yang Meng1, y 1Department of Physics and HKU-UCAS Joint Institute of Theoretical and Computational Physics, The University of Hong Kong, Pokfulam Road, Hong Kong 2School of Materials Science and Physics, China University of Mining and Technology, Xuzhou 221116, China 3School of Physics, Key Laboratory of Micro-Nano Measurement-Manipulation and Physics, Beihang University, Beijing 100191, China 4State Key Laboratory of Surface Physics, Fudan University, Shanghai 200433, China 5Center for Field Theory and Particle Physics, Department of Physics, Fudan University, Shanghai 200433, China 6Collaborative Innovation Center of Advanced Microstructures, Nanjing 210093, China Among the quantum many-body models that host anyon excitation and topological orders, quantum dimer models (QDM) provide a unique playground for studying the relation between single-anyon and multi-anyon continuum spectra. However, as the prototypical correlated system with local constraints, the generic solution of QDM at different lattice geometry and parameter regimes is still missing due to the lack of controlled methodologies. Here we obtain, via the newly developed sweeping cluster quantum Monte Carlo algorithm, the excitation spectra in different phases of the triangular lattice QDM. Our resultsp revealp the single vison excitations inside the /2 quantum spin liquid (QSL) and its condensation towards the 12 × 12 valence bond solid (VBS), and demonstrate the translational symmetry fractionalization and emergent O(4) symmetry at the QSL-VBS transition. We find the single vison excitations, whose convolution qualitatively reproduces the dimer spectra, are not free but subject to interaction effects throughout the transition. -

Polar Covalent Bonds: Electronegativity
Polar Covalent Bonds: Electronegativity Covalent bonds can have ionic character These are polar covalent bonds Bonding electrons attracted more strongly by one atom than by the other Electron distribution between atoms is not symmetrical Bond Polarity and Electronegativity Symmetrical Covalent Bonds Polar Covalent Bonds C – C + - C – H C – O (non-polar) (polar) Electronegativity (EN): intrinsic ability of an atom to attract the shared electrons in a covalent bond Inductive Effect: shifting of sigma bonded electrons in resppygonse to nearby electronegative atom The Periodic Table and Electronegativity C – H C - Br and C - I (non-pol)lar) (po l)lar) Bond Polarity and Inductive Effect Nonpolar Covalent Bonds: atoms with similar EN Polar Covalent Bonds: Difference in EN of atoms < 2 Ionic Bonds: Difference in EN > 2 C–H bonds, relatively nonpolar C-O, C-X bonds (more electronegative elements) are polar Bonding electrons shift toward electronegative atom C acqqppuires partial positive char g,ge, + Electronegative atom acquires partial negative charge, - Inductive effect: shifting of electrons in a bond in response to EN of nearby atoms Electrostatic Potential Maps Electrostatic potential maps show calculated charge distributions Colors indicate electron- rich (red) and electron- poor (blue ) reg ions Arrows indicate direction of bond polarity Polar Covalent Bonds: Net Dipole Moments Molecules as a whole are often polar from vector summation of individual bond polarities and lone-pair contributions Strongly polar substances soluble in polar solvents like water; nonpolar substances are insoluble in water. Dipole moment ( ) - Net molecular polarity, due to difference in summed charges - magnitude of charge Q at end of molecular dipole times distance r between charges = Q r, in debyes (D), 1 D = 3.336 1030 coulomb meter length of an average covalent bond, the dipole moment would be 1.60 1029 Cm, or 4.80 D. -

Heterocycles 2 Daniel Palleros
Heterocycles 2 Daniel Palleros Heterocycles 1. Structures 2. Aromaticity and Basicity 2.1 Pyrrole 2.2 Imidazole 2.3 Pyridine 2.4 Pyrimidine 2.5 Purine 3. Π-excessive and Π-deficient Heterocycles 4. Electrophilic Aromatic Substitution 5. Oxidation-Reduction 6. DNA and RNA Bases 7. Tautomers 8. H-bond Formation 9. Absorption of UV Radiation 10. Reactions and Mutations Heterocycles 3 Daniel Palleros Heterocycles Heterocycles are cyclic compounds in which one or more atoms of the ring are heteroatoms: O, N, S, P, etc. They are present in many biologically important molecules such as amino acids, nucleic acids and hormones. They are also indispensable components of pharmaceuticals and therapeutic drugs. Caffeine, sildenafil (the active ingredient in Viagra), acyclovir (an antiviral agent), clopidogrel (an antiplatelet agent) and nicotine, they all have heterocyclic systems. O CH3 N HN O O N O CH 3 N H3C N N HN N OH O S O H N N N 2 N O N N O CH3 N CH3 caffeine sildenafil acyclovir Cl S N CH3 N N H COOCH3 nicotine (S)-clopidogrel Here we will discuss the chemistry of this important group of compounds beginning with the simplest rings and continuing to more complex systems such as those present in nucleic acids. Heterocycles 4 Daniel Palleros 1. Structures Some of the most important heterocycles are shown below. Note that they have five or six-membered rings such as pyrrole and pyridine or polycyclic ring systems such as quinoline and purine. Imidazole, pyrimidine and purine play a very important role in the chemistry of nucleic acids and are highlighted. -
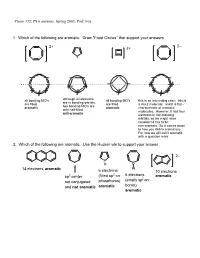
PS 6 Answers, Spring 2003, Prof
Chem 332, PS 6 answers, Spring 2003, Prof. Fox 1. Which of the following are aromatic. Draw "Frost Circles" that support your answers 2– 2+ 2+ + all bonding MO's although all electrons all bonding MO's this is an interesting case: this is are in bonding orbitals, are filled are filled a 4n+2 molecule, and it is flat-- two bonding MO's are aromatic aromatic characteristic of aromatic only half-filled molecules. However, it has four anti-aromatic electrons in non-bonding orbitals, so we might have considered this to be non-aromatic. So it comes down to how you define aromaticity. For now we will call it aromatic with a question mark. 2. Which of the following are aromatic. Use the Huckel rule to support your answer 2– P H B 14 electrons, aromatic H 6 electrons 10 electrons 2 6 electrons sp3 center (filled sp on aromatic (empty sp2 on not conjugated phosphorus) boron) and not aromatic aromatic aromatic 3. Compound 1 has an unusually large dipole moment for an organic hydrocarbon. Explain why (consider resonance structures for the central double bond) We need to remember that for any double bond compound, we can draw two polar resonance structure. Normally, these resonance structures are unimportant unimportant unimportant However, for 1 the story is different because there is a polar resonance form that has two aromatic components. In other words, 'breaking' the double bond gives rise to aromaticity, and therefore the polar resonance structure is important and leads to the observed dipole. dipole 1 8 e– 4 e– 6 e– 6 e– anti- anti- aromatic aromatic aromatic aromatic important resonance unimportant resonance structure structure 5. -

Structure of Benzene, Orbital Picture, Resonance in Benzene, Aromatic Characters, Huckel’S Rule B
Dr.Mrityunjay Banerjee, IPT, Salipur B PHARM 3rd SEMESTER BP301T PHARMACEUTICAL ORGANIC CHEMISTRY –II UNIT I UNIT I Benzene and its derivatives 10 Hours A. Analytical, synthetic and other evidences in the derivation of structure of benzene, Orbital picture, resonance in benzene, aromatic characters, Huckel’s rule B. Reactions of benzene - nitration, sulphonation, halogenationreactivity, Friedelcrafts alkylation- reactivity, limitations, Friedelcrafts acylation. C. Substituents, effect of substituents on reactivity and orientation of mono substituted benzene compounds towards electrophilic substitution reaction D. Structure and uses of DDT, Saccharin, BHC and Chloramine Benzene and its Derivatives Chemists have found it useful to divide all organic compounds into two broad classes: aliphatic compounds and aromatic compounds. The original meanings of the words "aliphatic" (fatty) and "aromatic" (fragrant/ pleasant smell). Aromatic compounds are benzene and compounds that resemble benzene in chemical behavior. Aromatic properties are those properties of benzene that distinguish it from aliphatic hydrocarbons. Benzene: A liquid that smells like gasoline Boils at 80°C & Freezes at 5.5°C It was formerly used to decaffeinate coffee and component of many consumer products, such as paint strippers, rubber cements, and home dry-cleaning spot removers. A precursor in the production of plastics (such as Styrofoam and nylon), drugs, detergents, synthetic rubber, pesticides, and dyes. It is used as a solvent in cleaning and maintaining printing equipment and for adhesives such as those used to attach soles to shoes. Benzene is a natural constituent of petroleum products, but because it is a known carcinogen, its use as an additive in gasoline is now limited. In 1970s it was associated with leukemia deaths. -
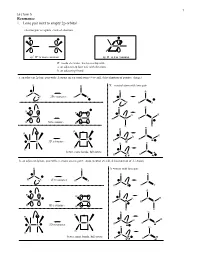
Resonance 1. Lone Pair Next to Empty 2P Orbital
1 Lecture 5 Resonance 1. Lone pair next to empty 2p orbital electron pair acceptors - lack of electrons C C CC sp2 R+ is more common sp R+ is less common R+ needs electrons, has to overlap with a. an adjacent 2p lone pair with electrons b. an adjacent pi bond a. an adjacent 2p lone pair with electrons on a neutral atom (+ overall, delocalization of positive charge) R R X = neutral atom with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R N R N R 3D resonance R R R R R R R C R C R CX R O CX 3D resonance R O R R R R C better, more bonds, full octets C R F R F b. an adjacent 2p lone pair with electrons on a negative atom (neutral overall, delocalization of electrons) R R X =anion with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R C R C R 3D resonance R R R R R R R C R C R CX R N CX 3D resonance R N R R R R C C better, more bonds, full octets R O R O 2 Lecture 5 Problem 1 – All of the following examples demonstrate delocalization of a lone pair of electrons into an empty 2p orbital. Usually in organic chemistry this is a carbocation site, but not always. -

Observing Spinons and Holons in 1D Antiferromagnets Using Resonant
Summary on “Observing spinons and holons in 1D antiferromagnets using resonant inelastic x-ray scattering.” Umesh Kumar1,2 1 Department of Physics and Astronomy, The University of Tennessee, Knoxville, TN 37996, USA 2 Joint Institute for Advanced Materials, The University of Tennessee, Knoxville, TN 37996, USA (Dated Jan 30, 2018) We propose a method to observe spinon and anti-holon excitations at the oxygen K-edge of Sr2CuO3 using resonant inelastic x-ray scattering (RIXS). The evaluated RIXS spectra are rich, containing distinct two- and four-spinon excitations, dispersive antiholon excitations, and combinations thereof. Our results further highlight how RIXS complements inelastic neutron scattering experiments by accessing charge and spin components of fractionalized quasiparticles Introduction:- One-dimensional (1D) magnetic systems are an important playground to study the effects of quasiparticle fractionalization [1], defined below. Hamiltonians of 1D models can be solved with high accuracy using analytical and numerical techniques, which is a good starting point to study strongly correlated systems. The fractionalization in 1D is an exotic phenomenon, in which electronic quasiparticle excitation breaks into charge (“(anti)holon”), spin (“spinon”) and orbit (“orbiton”) degree of freedom, and are observed at different characteristic energy scales. Spin-charge and spin-orbit separation have been observed using angle-resolved photoemission spectroscopy (ARPES) [2] and resonant inelastic x-ray spectroscopy (RIXS) [1], respectively. RIXS is a spectroscopy technique that couples to spin, orbit and charge degree of freedom of the materials under study. Unlike spin-orbit, spin-charge separation has not been observed using RIXS to date. In our work, we propose a RIXS experiment that can observe spin-charge separation at the oxygen K-edge of doped Sr2CuO3, a prototype 1D material. -

Topological Quantum Compiling Layla Hormozi
Florida State University Libraries Electronic Theses, Treatises and Dissertations The Graduate School 2007 Topological Quantum Compiling Layla Hormozi Follow this and additional works at the FSU Digital Library. For more information, please contact [email protected] THE FLORIDA STATE UNIVERSITY COLLEGE OF ARTS AND SCIENCES TOPOLOGICAL QUANTUM COMPILING By LAYLA HORMOZI A Dissertation submitted to the Department of Physics in partial fulfillment of the requirements for the degree of Doctor of Philosophy Degree Awarded: Fall Semester, 2007 The members of the Committee approve the Dissertation of Layla Hormozi defended on September 20, 2007. Nicholas E. Bonesteel Professor Directing Dissertation Philip L. Bowers Outside Committee Member Jorge Piekarewicz Committee Member Peng Xiong Committee Member Kun Yang Committee Member Approved: Mark A. Riley , Chair Department of Physics Joseph Travis , Dean, College of Arts and Sciences The Office of Graduate Studies has verified and approved the above named committee members. ii ACKNOWLEDGEMENTS To my advisor, Nick Bonesteel, I am indebted at many levels. I should first thank him for introducing me to the idea of topological quantum computing, for providing me with the opportunity to work on the problems that are addressed in this thesis, and for spending an infinite amount of time helping me toddle along, every step of the process, from the very beginning up until the completion of this thesis. I should also thank him for his uniquely caring attitude, for his generous support throughout the years, and for his patience and understanding for an often-recalcitrant graduate student. Thank you Nick — I truly appreciate all that you have done for me. -

Charge and Spin Fractionalization in Strongly Correlated Topological Insulators
Charge and spin fractionalization in strongly correlated topological insulators Predrag Nikolić George Mason University October 26, 2011 Acknowledgments Zlatko Tešanović Michael Levin Tanja Duric IQM @ Johns Hopkins University of Maryland Max Planck Institute, Dresden • Affiliations and sponsors The Center for Quantum Science Charge and spin fractionalization in strongly correlated topological insulators 2/33 Overview • TIs with time-reversal symmetry – Introduction to topological band-insulators – Introduction to interacting TIs • Experimental realization of strongly correlated TIs – Cooper pair TI by proximity effect – Exciton TI • Theory of strongly correlated TIs – Topological Landau-Ginzburg theory – Charge and spin fractionalization Charge and spin fractionalization in strongly correlated topological insulators 3/33 Topological vs. Conventional • Conventional states of matter – Characterized by local properties (LDOS, order parameter...) • Topological states of matter – Characterized by non-local properties (entanglement, edge modes, torus spectra...) • Examples of topological quantum states – Quantum Hall states & “topological insulators” – Spin liquids, string-net condensates... Charge and spin fractionalization in strongly correlated topological insulators 4/33 The Appeal of Topological • Macroscopic quantum entanglement – What makes quantum mechanics fascinating... – Still uncharted class of quantum states • Non-local properties are hard to perturb – Standard for measurements of resistivity – Topological quantum computation • -

Physical Organic Chemistry
PHYSICAL ORGANIC CHEMISTRY Yu-Tai Tao (陶雨台) Tel: (02)27898580 E-mail: [email protected] Website:http://www.sinica.edu.tw/~ytt Textbook: “Perspective on Structure and Mechanism in Organic Chemistry” by F. A. Corroll, 1998, Brooks/Cole Publishing Company References: 1. “Modern Physical Organic Chemistry” by E. V. Anslyn and D. A. Dougherty, 2005, University Science Books. Grading: One midterm (45%) one final exam (45%) and 4 quizzes (10%) homeworks Chap.1 Review of Concepts in Organic Chemistry § Quantum number and atomic orbitals Atomic orbital wavefunctions are associated with four quantum numbers: principle q. n. (n=1,2,3), azimuthal q.n. (m= 0,1,2,3 or s,p,d,f,..magnetic q. n. (for p, -1, 0, 1; for d, -2, -1, 0, 1, 2. electron spin q. n. =1/2, -1/2. § Molecular dimensions Atomic radius ionic radius, ri:size of electron cloud around an ion. covalent radius, rc:half of the distance between two atoms of same element bond to each other. van der Waal radius, rvdw:the effective size of atomic cloud around a covalently bonded atoms. - Cl Cl2 CH3Cl Bond length measures the distance between nucleus (or the local centers of electron density). Bond angle measures the angle between lines connecting different nucleus. Molecular volume and surface area can be the sum of atomic volume (or group volume) and surface area. Principle of additivity (group increment) Physical basis of additivity law: the forces between atoms in the same molecule or different molecules are very “short range”. Theoretical determination of molecular size:depending on the boundary condition. -

Localized Electron Model for Bonding What Does Hybridization Mean?
190 Localized electron model for bonding 1. Determine the Lewis Structure for the molecule 2. Determine resonance structures 3. Determine the shape of the molecule 4. Determine the hybridization of the atoms What does hybridization mean? Why hybridize? Look at the shape of many molecules, and compare this to the shape of the orbitals of each of the atoms. Shape of orbitals Orbitals on atoms are s, p, d, and f, and there are certain shapes associated with each orbital. an s orbital is a big sphere the f orbitals look like two four leaf the p orbitals looks like “8” ’s clovers each one points in along an axis three f orbitals look like weird “8”’s with two doughnuts around each four of the d orbitals look like four one leaf clovers one d orbital looks like an “8” with a doughnut around the middle Shape of molecules Remember we determine the shape of molecules by determining the number of sets of electrons around the atom. count double and triple bonds as one set, count single bonds as one set of electrons and count lone pairs as a set 2 sets—each pair of electrons points 180° away from the other 5 sets—each pair points toward the corner of a trigonal bipyramid 3 sets—each pair points toward the corner of a triangle 6 sets—each pair points toward the corners of an octahedron 4 sets—each pair points toward the corner of a tetrahedron 191 To make bonds we need orbitals—the electrons need to go some where. -

Spin Liquids with Topological Order
Spin Liquids with Topological Order 2d Mott Insulators with one electron per unit cell Quantum s=1/2 magnets Will show that topologically ordered spin liquids have: • an emergent “gauge structure” • Quantum number fractionalization • Ground state degeneracy on a torus Focus on Spin liquids with: • Z2 Topological order • Fully gapped with bosonic “spinons” and “visons” • 2d square lattice and Kagome lattice Resonating Valence Bond “Picture” 2d square lattice s=1/2 AFM = Singlet or a Valence Bond - Gains exchange energy J Valence Bond Solid Plaquette Resonance Resonating Valence Bond “Spin liquid” Plaquette Resonance Resonating Valence Bond “Spin liquid” Plaquette Resonance Resonating Valence Bond “Spin liquid” Gapped Spin Excitations “Break” a Valence Bond - costs energy of order J Create s=1 excitation Try to separate two s=1/2 “spinons” Valence Bond Solid Energy cost is linear in separation Spinons are “Confined” in VBS RVB State: Exhibits Fractionalization! Energy cost stays finite when spinons are separated Spinons are “deconfined” in the RVB state Spinon carries the electrons spin, but not its charge ! The electron is “fractionalized”. Gauge Theory Formulation of RVB Spin liquid Focus on the Valence bonds and their quantum dynamics - “A Quantum Dimer model” Place a “spin” on each link i j of the lattice vector of Pauli matrices no bond on link ij Define: Plaquette “Flux” bond on link ij 1 -1 -1 1 Hamiltonian: Need a Constraint: One valence bond coming out of each site Z2 Gauge Theory: Qi = -1 implies one or three bonds out of each