Expressing SI Units
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix A: Symbols and Prefixes
Appendix A: Symbols and Prefixes (Appendix A last revised November 2020) This appendix of the Author's Kit provides recommendations on prefixes, unit symbols and abbreviations, and factors for conversion into units of the International System. Prefixes Recommended prefixes indicating decimal multiples or submultiples of units and their symbols are as follows: Multiple Prefix Abbreviation 1024 yotta Y 1021 zetta Z 1018 exa E 1015 peta P 1012 tera T 109 giga G 106 mega M 103 kilo k 102 hecto h 10 deka da 10-1 deci d 10-2 centi c 10-3 milli m 10-6 micro μ 10-9 nano n 10-12 pico p 10-15 femto f 10-18 atto a 10-21 zepto z 10-24 yocto y Avoid using compound prefixes, such as micromicro for pico and kilomega for giga. The abbreviation of a prefix is considered to be combined with the abbreviation/symbol to which it is directly attached, forming with it a new unit symbol, which can be raised to a positive or negative power and which can be combined with other unit abbreviations/symbols to form abbreviations/symbols for compound units. For example: 1 cm3 = (10-2 m)3 = 10-6 m3 1 μs-1 = (10-6 s)-1 = 106 s-1 1 mm2/s = (10-3 m)2/s = 10-6 m2/s Abbreviations and Symbols Whenever possible, avoid using abbreviations and symbols in paragraph text; however, when it is deemed necessary to use such, define all but the most common at first use. The following is a recommended list of abbreviations/symbols for some important units. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

SI and CGS Units in Electromagnetism
SI and CGS Units in Electromagnetism Jim Napolitano January 7, 2010 These notes are meant to accompany the course Electromagnetic Theory for the Spring 2010 term at RPI. The course will use CGS units, as does our textbook Classical Electro- dynamics, 2nd Ed. by Hans Ohanian. Up to this point, however, most students have used the International System of Units (SI, also known as MKSA) for mechanics, electricity and magnetism. (I believe it is easy to argue that CGS is more appropriate for teaching elec- tromagnetism to physics students.) These notes are meant to smooth the transition, and to augment the discussion in Appendix 2 of your textbook. The base units1 for mechanics in SI are the meter, kilogram, and second (i.e. \MKS") whereas in CGS they are the centimeter, gram, and second. Conversion between these base units and all the derived units are quite simply given by an appropriate power of 10. For electromagnetism, SI adds a new base unit, the Ampere (\A"). This leads to a world of complications when converting between SI and CGS. Many of these complications are philosophical, but this note does not discuss such issues. For a good, if a bit flippant, on- line discussion see http://info.ee.surrey.ac.uk/Workshop/advice/coils/unit systems/; for a more scholarly article, see \On Electric and Magnetic Units and Dimensions", by R. T. Birge, The American Physics Teacher, 2(1934)41. Electromagnetism: A Preview Electricity and magnetism are not separate phenomena. They are different manifestations of the same phenomenon, called electromagnetism. One requires the application of special relativity to see how electricity and magnetism are united. -
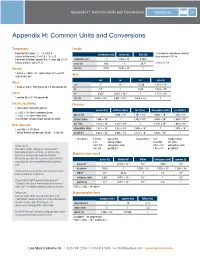
Appendix H: Common Units and Conversions Appendices 215
Appendix H: Common Units and Conversions Appendices 215 Appendix H: Common Units and Conversions Temperature Length Fahrenheit to Celsius: °C = (°F-32)/1.8 1 micrometer (sometimes referred centimeter (cm) meter (m) inch (in) Celsius to Fahrenheit: °F = (1.8 × °C) + 32 to as micron) = 10-6 m Fahrenheit to Kelvin: convert °F to °C, then add 273.15 centimeter (cm) 1 1.000 × 10–2 0.3937 -3 Celsius to Kelvin: add 273.15 meter (m) 100 1 39.37 1 mil = 10 in Volume inch (in) 2.540 2.540 × 10–2 1 –3 3 1 liter (l) = 1.000 × 10 cubic meters (m ) = 61.02 Area cubic inches (in3) cm2 m2 in2 circ mil Mass cm2 1 10–4 0.1550 1.974 × 105 1 kilogram (kg) = 1000 grams (g) = 2.205 pounds (lb) m2 104 1 1550 1.974 × 109 Force in2 6.452 6.452 × 10–4 1 1.273 × 106 1 newton (N) = 0.2248 pounds (lb) circ mil 5.067 × 10–6 5.067 × 10–10 7.854 × 10–7 1 Electric resistivity Pressure 1 micro-ohm-centimeter (µΩ·cm) pascal (Pa) millibar (mbar) torr (Torr) atmosphere (atm) psi (lbf/in2) = 1.000 × 10–6 ohm-centimeter (Ω·cm) –2 –3 –6 –4 = 1.000 × 10–8 ohm-meter (Ω·m) pascal (Pa) 1 1.000 × 10 7.501 × 10 9.868 × 10 1.450 × 10 = 6.015 ohm-circular mil per foot (Ω·circ mil/ft) millibar (mbar) 1.000 × 102 1 7.502 × 10–1 9.868 × 10–4 1.450 × 10–2 2 0 –3 –2 Heat flow rate torr (Torr) 1.333 × 10 1.333 × 10 1 1.316 × 10 1.934 × 10 5 3 2 1 1 watt (W) = 3.413 Btu/h atmosphere (atm) 1.013 × 10 1.013 × 10 7.600 × 10 1 1.470 × 10 1 British thermal unit per hour (Btu/h) = 0.2930 W psi (lbf/in2) 6.897 × 103 6.895 × 101 5.172 × 101 6.850 × 10–2 1 1 torr (Torr) = 133.332 pascal (Pa) 1 pascal (Pa) = 0.01 millibar (mbar) 1.33 millibar (mbar) 0.007501 torr (Torr) –6 A Note on SI 0.001316 atmosphere (atm) 9.87 × 10 atmosphere (atm) 2 –4 2 The values in this catalog are expressed in 0.01934 psi (lbf/in ) 1.45 × 10 psi (lbf/in ) International System of Units, or SI (from the Magnetic induction B French Le Système International d’Unités). -

Physics, Chapter 30: Magnetic Fields of Currents
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Robert Katz Publications Research Papers in Physics and Astronomy 1-1958 Physics, Chapter 30: Magnetic Fields of Currents Henry Semat City College of New York Robert Katz University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/physicskatz Part of the Physics Commons Semat, Henry and Katz, Robert, "Physics, Chapter 30: Magnetic Fields of Currents" (1958). Robert Katz Publications. 151. https://digitalcommons.unl.edu/physicskatz/151 This Article is brought to you for free and open access by the Research Papers in Physics and Astronomy at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Robert Katz Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 30 Magnetic Fields of Currents 30-1 Magnetic Field around an Electric Current The first evidence for the existence of a magnetic field around an electric current was observed in 1820 by Hans Christian Oersted (1777-1851). He found that a wire carrying current caused a freely pivoted compass needle B D N rDirection I of current D II. • ~ I I In wire ' \ N I I c , I s c (a) (b) Fig. 30-1 Oersted's experiment. Compass needle is deflected toward the west when the wire CD carrying current is placed above it and the direction of the current is toward the north, from C to D. in its vicinity to be deflected. If the current in a long straight wire is directed from C to D, as shown in Figure 30-1, a compass needle below it, whose initial orientation is shown in dotted lines, will have its north pole deflected to the left and its south pole deflected to the right. -

CAR-ANS PART 05 Issue No. 2 Units of Measurement to Be Used In
CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES Part 5 Governing UNITS OF MEASUREMENT TO BE USED IN AIR AND GROUND OPERATIONS CIVIL AVIATION AUTHORITY OF THE PHILIPPINES Old MIA Road, Pasay City1301 Metro Manila INTENTIONALLY LEFT BLANK CAR-ANS PART 5 Republic of the Philippines CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES (CAR-ANS) Part 5 UNITS OF MEASUREMENTS TO BE USED IN AIR AND GROUND OPERATIONS 22 APRIL 2016 EFFECTIVITY Part 5 of the Civil Aviation Regulations-Air Navigation Services are issued under the authority of Republic Act 9497 and shall take effect upon approval of the Board of Directors of the CAAP. APPROVED BY: LT GEN WILLIAM K HOTCHKISS III AFP (RET) DATE Director General Civil Aviation Authority of the Philippines Issue 2 15-i 16 May 2016 CAR-ANS PART 5 FOREWORD This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was formulated and issued by the Civil Aviation Authority of the Philippines (CAAP), prescribing the standards and recommended practices for units of measurements to be used in air and ground operations within the territory of the Republic of the Philippines. This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was developed based on the Standards and Recommended Practices prescribed by the International Civil Aviation Organization (ICAO) as contained in Annex 5 which was first adopted by the council on 16 April 1948 pursuant to the provisions of Article 37 of the Convention of International Civil Aviation (Chicago 1944), and consequently became applicable on 1 January 1949. The provisions contained herein are issued by authority of the Director General of the Civil Aviation Authority of the Philippines and will be complied with by all concerned. -

Magnetic Units
Magnetic Units Magnetic poles, moments and magnetic dipoles The famous inverse square force law between two poles p1 and p2 separated by a distance r was discovered by the English scientist John Michell (1750), and the French scientist Charles Coulomb (1785): In the cgs (electromagnetic) system, k = 1, and the interaction force between two poles each of unit strength (in emu) separated by 1 cm is equal to 1 dyne. Alternatively, one can visualize this force as an interaction force between the pole p0 and the field H produced by another other pole of strength p: Therefore, the field produced by p at the location of p0 a distance r away is given by: The unit of the magnetic field, the Oersted (Oe), is defined as the strength of the field produced by a unit pole at a point 1 cm away (by eq. 3). Alternatively, it is the strength of the field which exerts a force of 1 dyne of a unit pole (by eq. 2). Faraday’s representation Michael Faraday represented the field strength in terms of “lines of force” (1 line of force = 1 maxwell (= 1 Mx)). In this representation, the field strength is defined as the number of lines of force passing through a unit area normal to the field. Therefore: 1 Oe = 1 line of force/cm2 = 1 Mx/cm2 (4) A bar magnet has a magnetic moment given in terms of the pole strength and length of the magnet by: The unit of the magnetic moment in cgs system is emu = erg/Oe. It is worth mentioning that the magnetic pole does not exist, neither can the distance between the two poles be determined accurately due to non-localization of the pole. -

The International System of Units (SI) - Conversion Factors For
NIST Special Publication 1038 The International System of Units (SI) – Conversion Factors for General Use Kenneth Butcher Linda Crown Elizabeth J. Gentry Weights and Measures Division Technology Services NIST Special Publication 1038 The International System of Units (SI) - Conversion Factors for General Use Editors: Kenneth S. Butcher Linda D. Crown Elizabeth J. Gentry Weights and Measures Division Carol Hockert, Chief Weights and Measures Division Technology Services National Institute of Standards and Technology May 2006 U.S. Department of Commerce Carlo M. Gutierrez, Secretary Technology Administration Robert Cresanti, Under Secretary of Commerce for Technology National Institute of Standards and Technology William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publications 1038 Natl. Inst. Stand. Technol. Spec. Pub. 1038, 24 pages (May 2006) Available through NIST Weights and Measures Division STOP 2600 Gaithersburg, MD 20899-2600 Phone: (301) 975-4004 — Fax: (301) 926-0647 Internet: www.nist.gov/owm or www.nist.gov/metric TABLE OF CONTENTS FOREWORD.................................................................................................................................................................v -

Abampere (Ab-AM-Peer)—The Cgs Electromagnetic Unit of Electric Current Equivalent to 10 Amperes
HEAVA1p.qxd 7/16/1 9:59 AM Page 1 A AA B abampere (ab-AM-peer)—the cgs electromagnetic unit of electric current equivalent to 10 amperes. abdomen (AB-duh-men)—the belly; the cavity in the body between the thorax and the pelvis that contains the stomach, intestines, liver, and kidneys. abducent (ab-DOOS-int)—drawing away from, as muscles draw away. abducent nerve (ab-DOOS-int NURV)—either of the six cranial nerves; small motor nerves supplying the external rectus muscle of the eye. abductor (ab-DUK-tur)—muscles that separate the fingers. Opposite of adductor. abductor hallucis (ab-DUK-tor huh-LOO-sis)—a muscle of the great toe. ability (uh-BIL-ih-tee)—the quality or state of being able to perform. abiogenesis (ay-by-oh-JEN-uh-sus)—the generating or springing up of living from nonliving matter; spontaneous generation. abiosis (ab-ee-OH-sis)—absence of life. abirritant (ab-IHR-uh-tant)—a soothing agent that relieves irritation. abnormal (ab-NOR-mul)—irregular; contrary to the natural law or customary order. abnormality (ab-nor-MAL-ih-tee)—the state or condition of being ab- normal or unusual. abohm (ab-OHM)—the cgs electromagnetic unit of resistance equal to one millionth of an ohm. aboral (ah-BOHR-ul)—located or situated opposite to or away from the mouth. abrade (uh-BRAYD)—to remove or roughen by friction or rubbing. 1 HEAVA1p.qxd 7/16/1 9:59 AM Page 2 2 abrasion abrasion (uh-BRAY-zhun)—scraping of the skin; excoriation; rubbing A or wearing off of the surface; an irritation; a scraped or B scratched area of the skin. -

Units of Measurement to Be Used in Air and Ground Operations
International Standards and Recommended Practices Annex 5 to the Convention on International Civil Aviation Units of Measurement to be Used in Air and Ground Operations This edition incorporates all amendments adopted by the Council prior to 23 February 2010 and supersedes, on 18 November 2010, all previous editions of Annex 5. For information regarding the applicability of the Standards and Recommended Practices,see Foreword. Fifth Edition July 2010 International Civil Aviation Organization Suzanne TRANSMITTAL NOTE NEW EDITIONS OF ANNEXES TO THE CONVENTION ON INTERNATIONAL CIVIL AVIATION It has come to our attention that when a new edition of an Annex is published, users have been discarding, along with the previous edition of the Annex, the Supplement to the previous edition. Please note that the Supplement to the previous edition should be retained until a new Supplement is issued. Suzanne International Standards and Recommended Practices Annex 5 to the Convention on International Civil Aviation Units of Measurement to be Used in Air and Ground Operations ________________________________ This edition incorporates all amendments adopted by the Council prior to 23 February 2010 and supersedes, on 18 November 2010, all previous editions of Annex 5. For information regarding the applicability of the Standards and Recommended Practices, see Foreword. Fifth Edition July 2010 International Civil Aviation Organization Published in separate English, Arabic, Chinese, French, Russian and Spanish editions by the INTERNATIONAL CIVIL AVIATION ORGANIZATION 999 University Street, Montréal, Quebec, Canada H3C 5H7 For ordering information and for a complete listing of sales agents and booksellers, please go to the ICAO website at www.icao.int First edition 1948 Fourth edition 1979 Fifth edition 2010 Annex 5, Units of Measurement to be Used in Air and Ground Operations Order Number: AN 5 ISBN 978-92-9231-512-2 © ICAO 2010 All rights reserved. -

Units of Measure
Units of Measure Units marked with asterisks are base, derived, or supplementary units of the Systeme International. Unit Abbreviation abampere - spell out abohm - spell out abvolt - spell out amagat - spell out *ampere - A ampere hour - A h ampere turns per meter - At/m angstrom - Å arc minute - arc min astronomical unit - AU atmosphere - atm atmosphere, standard - As atomic mass unit - u atomic parts per million - at. ppm atomic percent - at. % atomic time unit - atu atomic unit- a.u. attofarad - aF bar - spell out bark - spell out barn - b barye - spell out biot - Bi bit or bits - spell out blobs per hundred microns - blobs/(100 um) bohr - spell out British thermal unit - Btu bytes - spell out calorie - cal *candela - cd candelas per square meter - cd/m2 candlepower - cp centimeter - cm centipoise - cP *coulomb - C counts per minute - counts/min, cpm counts per second - counts/s cubic centimeter - cm3, (cc not rec.) curie - Ci cycle - spell out, c cycles per second - cps, c/s day d, - or spell out debye - D decibel - dB, dBm degree - [ring], deg degrees - Baumé [ring]B degrees - Celsius (centigrade) [ring]C degrees - Fahrenheit [ring]F degrees - Kelvin K disintegrations per minute - dis/min disintegrations per minute per microgram - dis/min ug disintegrations per second - dis/s dyne - dyn electromagnetic unit - emu electron barn - eb electrons per atom - e/at. electrons per cubic centimeter - e/cm3, e/cc, e cm-3 electron unit - e.u. electron volt - eV electrostatic unit - esu entropy unit - eu erg - spell out *farad - F femtofarad - fF femtometer - fm fermi - F fissions per minute - fpm foot - ft foot-candle - fc foot-lambert - fL foot-pound - ft lb formula units - f.u. -

Conversions of D and H Between SI and Gaussian Units 1 Problem
Conversions of D and H between SI and Gaussian Units Kirk T. McDonald Joseph Henry Laboratories, Princeton University, Princeton, NJ 08544 (April 12, 2014; updated April 23, 2014) 1Problem The electric displacement field D has dimensions charge/area and the magnetic field strength H has dimensions current/length. In the SI system the units of D are C(oulomb)/m2 and the units of H are A/m, while in the Gaussian (cgs) system the units of D are sC(statcoulomb)/cm2 and the units of H are Oe (oersteds) where1,2,3 1Oe=ccgs sA(statampere)/cm (= 1 abampere/cm in the EMU system), (1) and c is the speed of light in vacuum. The conversions of coulombs to statcoulombs, of amperes to statamperes, are that [1, 10, 11], c c 1C= cgs sC ≈ 3 × 109 sC, and 1 A = cgs sA ≈ 3 × 109 sA. (2) 10 10 The conversion of D is that [10, 11], 2 −5 2 5 2 DSI =1C/m = Dcgs =4πccgs × 10 sC/cm ≈ 12π × 10 sC/cm , (3) and the conversion of H is that [1, 10, 11], −3 −3 7 HSI =1A/m=Hcgs =4π × 10 Oe = 4πccgs × 10 sA/cm ≈ 12π × 10 sA/cm. (4) However, from eq. (2) we find the conversions, 2 −5 2 −3 1C/m = ccgs × 10 sC/cm , and 1 A/m=ccgs × 10 sA/cm. (5) Could it be that the conversions (3)-(4) erroneously include factors of 4π? This problem was suggested by Neal Carron. 1 Strictly, the dimensions of Hcgs follow from Amp`ere’s law [1], ∇×Hcgs =4πIcgs/ccgs, as current/(length 2 2 · velocity), i.e.,sA·s/cm .