The International System of Units (SI) - Conversion Factors For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Difference Between BTU and Watts Key Difference - BTU Vs Watts
Difference Between BTU and Watts www.differencebetween.com Key Difference - BTU vs Watts It is first important to identify the concepts of energy and power in order to understand the difference between BTU and Watts. If an object is doing a work, the object is given an amount of energy to perform the task. If there is a heat transfer from or to an object, an amount of energy is removed from or given to the said object. The rate of work done or the rate of the heat transfer is defined as power. BTU and Watt are two different types of measurement units to measure the energy transfer and power, respectively. Thus, the key difference between BTU and Watts is that BTU measures energy, which is a stand-alone physical property whereas Watts measures the rate of transfer of energy that is always associated with a time factor. What is BTU? BTU is the abbreviated form for British Thermal Unit. The term thermal is often used for measuring thermal energy or the energy in the form of heat. BTU is not a part of the International System of Units or SI units. But it is often used as a measurement in heating and air-conditioning industry even at present. One BTU is defined as the amount of heat that should be transferred to one pound (lb) of water to raise its temperature by one degree of Fahrenheit (F). Since lb and F are both conventional units, the BTU can be identified by its SI units’ counterpart, Joule (J). That is, one joule is the heat required to transfer to one gram of water to raise the temperature by one degree Celsius (C). -

Soda Can Calorimeter
Soda Can Calorimeter Energy Content of Food SCIENTIFIC SCIENCEFAX! Introduction Have you ever noticed the nutrition label located on the packaging of the food you buy? One of the first things listed on the label are the calories per serving. How is the calorie content of food determined? This activity will introduce the concept of calorimetry and investigate the caloric content of snack foods. Concepts •Calorimetry • Conservation of energy • First law of thermodynamics Background The law of conservation of energy states that energy cannot be created or destroyed, only converted from one form to another. This fundamental law was used by scientists to derive new laws in the field of thermodynamics—the study of heat energy, temperature, and heat transfer. The First Law of Thermodynamics states that the heat energy lost by one body is gained by another body. Heat is the energy that is transferred between objects when there is a difference in temperature. Objects contain heat as a result of the small, rapid motion (vibrations, rotational motion, electron spin, etc.) that all atoms experience. The temperature of an object is an indirect measurement of its heat. Particles in a hot object exhibit more rapid motion than particles in a colder object. When a hot and cold object are placed in contact with one another, the faster moving particles in the hot object will begin to bump into the slower moving particles in the colder object making them move faster (vice versa, the faster particles will then move slower). Eventually, the two objects will reach the same equilibrium temperature—the initially cold object will now be warmer, and the initially hot object will now be cooler. -

Natural Gas, 22.6
Syllabus Section I Power Market Restructuring: Current Context and Historical Events ** Important Acknowledgement: These notes are an edited abridged version of a set of on-line lecture notes prepared by Professor Tom Overbye, ECpE, University of Illinois, 2008 Last Revised: 9/13/2011 Setting Course Topics in Context EE/Econ 458 focuses on the restructuring of wholesale power markets, using the U.S. as the primary source of illustrations. Particular attention will be focused on U.S. restructuring efforts in the Midwest (MISO), New England (ISO-NE), New York (NYISO), mid- Atlantic States (PJM), California (CAISO), Texas (ERCOT), and the Southwest Power Pool (SPP). First, however, it is important to consider this restructuring movement within the larger context of the energy delivery system as a whole. Electric Systems in Energy Context Electricity is used primarily as a means for energy transportation. – Other sources of energy are used to create it, and once created it is usually converted into other forms of energy before actual use. About 40% of US energy is transported in electric form. Concerns about CO2 emissions and the depletion of fossil fuels are becoming main drivers for change in the world energy infrastructure. Measurement of Power Power: Instantaneous consumption of energy Power Units Watts (W) = voltage times current for DC systems kW – 1 x 103 Watts MW – 1 x 106 Watts GW – 1 x 109 Watts Installed U.S. generation capacity is about 900 GW ( about 3 kW per person) Measurement of Energy Energy: Integration of power over time; energy is what people really want from a power system Energy Units: Joule = 1 Watt-second (J) kWh – Kilowatt-hour (3.6 x 106 J) Btu – 1054.85 J (British Thermal Unit) Note on Unit Conversion: 3413 Btu ≈ 1 kWh Annual U.S. -

Appendix A: Symbols and Prefixes
Appendix A: Symbols and Prefixes (Appendix A last revised November 2020) This appendix of the Author's Kit provides recommendations on prefixes, unit symbols and abbreviations, and factors for conversion into units of the International System. Prefixes Recommended prefixes indicating decimal multiples or submultiples of units and their symbols are as follows: Multiple Prefix Abbreviation 1024 yotta Y 1021 zetta Z 1018 exa E 1015 peta P 1012 tera T 109 giga G 106 mega M 103 kilo k 102 hecto h 10 deka da 10-1 deci d 10-2 centi c 10-3 milli m 10-6 micro μ 10-9 nano n 10-12 pico p 10-15 femto f 10-18 atto a 10-21 zepto z 10-24 yocto y Avoid using compound prefixes, such as micromicro for pico and kilomega for giga. The abbreviation of a prefix is considered to be combined with the abbreviation/symbol to which it is directly attached, forming with it a new unit symbol, which can be raised to a positive or negative power and which can be combined with other unit abbreviations/symbols to form abbreviations/symbols for compound units. For example: 1 cm3 = (10-2 m)3 = 10-6 m3 1 μs-1 = (10-6 s)-1 = 106 s-1 1 mm2/s = (10-3 m)2/s = 10-6 m2/s Abbreviations and Symbols Whenever possible, avoid using abbreviations and symbols in paragraph text; however, when it is deemed necessary to use such, define all but the most common at first use. The following is a recommended list of abbreviations/symbols for some important units. -

What Is a Calorie?
What Is A Calorie? Guide Copyright © 2008 Learning Seed Suite 301 641 West Lake Street Chicago, IL 60661 800.634.4941 [email protected] www.learningseed.com What Is A Calorie? Legal Niceties The Video Copyright © 2005 Learning Seed. This video program is protected under U.S. copyright law. No part of this video may be reproduced or transmitted by any means, electronic or mechanical, without the written permission of the Publisher, except where permitted by law. This Teaching Guide Copyright © 2008 Learning Seed. This teaching guide is copyrighted according to the terms of the Creative Commons non-commercial license (http://creativecommons.org/licenses/by-nc/2.5/). It may be reproduced, in its part or its entirety, for classroom use. No part of this guide may be reproduced for sale by any party. You are free: • to copy, distribute, display, and perform the work. • to make derivative works. Under the following conditions: • Attribution. You must attribute the work to Learning Seed. • Noncommercial. You may not use this work for commercial purposes. • For any reuse or distribution, you must make clear to others the license terms of this work. • Any of these conditions can be waived if you get permission from the copyright holder. Credits This Teaching Guide Compilation: Rebecca Phipps Copy Editor: Jennifer Smith Learning Seed Catalog and ISBN Numbers Please contact us with any questions or concerns at: VHS LS-1284-05-VHS ISBN 0-917159-75-6 Learning Seed DVD LS-1284-05-DVD ISBN 0-917159-23-3 Suite 301 641 West Lake Street Chicago, IL 60661 800.634.4941 [email protected] Closed Captioning This program is closed-captioned. -

Pressure, Its Units of Measure and Pressure References
_______________ White Paper Pressure, Its Units of Measure and Pressure References Viatran Phone: 1‐716‐629‐3800 3829 Forest Parkway Fax: 1‐716‐693‐9162 Suite 500 [email protected] Wheatfield, NY 14120 www.viatran.com This technical note is a summary reference on the nature of pressure, some common units of measure and pressure references. Read this and you won’t have to wait for the movie! PRESSURE Gas and liquid molecules are in constant, random motion called “Brownian” motion. The average speed of these molecules increases with increasing temperature. When a gas or liquid molecule collides with a surface, momentum is imparted into the surface. If the molecule is heavy or moving fast, more momentum is imparted. All of the collisions that occur over a given area combine to result in a force. The force per unit area defines the pressure of the gas or liquid. If we add more gas or liquid to a constant volume, then the number of collisions must increase, and therefore pressure must increase. If the gas inside the chamber is heated, the gas molecules will speed up, impact with more momentum and pressure increases. Pressure and temperature therefore are related (see table at right). The lowest pressure possible in nature occurs when there are no molecules at all. At this point, no collisions exist. This condition is known as a pure vacuum, or the absence of all matter. It is also possible to cool a liquid or gas until all molecular motion ceases. This extremely cold temperature is called “absolute zero”, which is -459.4° F. -
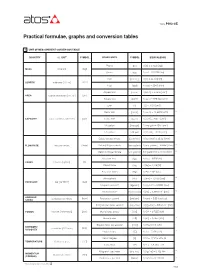
Practical Formulae, Graphs and Conversion Tables
Table P003-4/E Practical formulae, graphs and conversion tables 1 UNIT OF MEASUREMENT CONVERSION TABLE QUANTITY S.I. UNIT SYMBOL OTHER UNITS SYMBOL EQUIVALENCE Pound [lb] 1 [lb] = 0,4536 [kg] kilogram [kg] MASS Ounce [oz] 1 [oz] = 0,02335 [kg] Inch [in] or [”] 1 [in] = 25,40 [mm] millimeter [10-3 m] [mm] LENGTH Foot [foot] 1 [foot] = 304,8 [mm] Square inch [sq in] 1 [sq in] = 6,4516 [cm2] -4 2 [cm2] AREA square centimeter [10 m ] Square foot [sq ft] 1 [sq ft] = 929,034 [cm2] Liter [l] 1 [l] = 1000 [cm3] Cubic inch [cu in] 1 [cu in] = 16,3870 [cm3] * cubic centimeter [10-6 m3] [cm3] Cubic foot [cu ft] 1 [cu ft] = 28317 [cm3] CAPACITY UK gallon [Imp gal] 1 [Imp gal] = 4546 [cm3] US gallon [US gal] 1 [US gal] = 3785 [cm3] * Cubic foot per minute [cu ft/min] 1 [cu ft/min] = 28,32 [l/min] liter per minute [l/min] Gallon (UK) per minute [Imp gal/min] 1 [Imp gal/min] = 4,5456 [l/min] * FLOW RATE Gallon (US) per minute [US gal/min] [US gal/min] = 3,7848 [l/min] * Kilogram force [kgf] 1 [kgf] = 9,806 [N] Newton [kgm/s2] [N] FORCE Pound force [lbf] 1 [lbf] = 4,448 [N] Pascal [1 N/m2] [Pa] 1 [Pa] = 10-5 [bar] Atmosphere [atm] 1 [atm] = 1,0132 [bar] * bar [105 N/m2] [bar] PRESSURE 2 2 2 Kilogram force/cm [kgf/cm ] 1 [kgf/cm ] = 0,9806 [bar] 2 2 -2 Pound force/in [lbf /in ] or [psi] 1 [psi] = 6,8948•10 [bar] * ANGULAR revolution per minute [rpm] Radian per second [rad/sec] 1 [rpm] = 9,55 [rad/sec] SPEED -3 Kilogram per meter second [kgf •m/s] 1 [kgf •m/s] = 9,803•10 [kW] kilowatt [1000 Nm/s] [kW] Metric horse power [CV] 1 [CV] = 0,7355 [kW] POWER -

American and BRITISH UNITS of Measurement to SI UNITS
AMERICAN AND BRITISH UNITS OF MEASUREMENT TO SI UNITS UNIT & ABBREVIATION SI UNITS CONVERSION* UNIT & ABBREVIATION SI UNITS CONVERSION* UNITS OF LENGTH UNITS OF MASS 1 inch = 40 lines in 2.54 cm 0.393701 1 grain gr 64.7989 mg 0.0154324 1 mil 25.4 µm 0.03937 1 dram dr 1.77185 g 0.564383 1 line 0.635 mm 1.57480 1 ounce = 16 drams oz 28.3495 g 0.0352739 1 foot = 12 in = 3 hands ft 30.48 cm 0.0328084 1 pound = 16 oz lb 0.453592 kg 2.204622 1 yard = 3 feet = 4 spans yd 0.9144 m 1.09361 1 quarter = 28 lb 12.7006 kg 0.078737 1 fathom = 2 yd fath 1.8288 m 0.546807 1 hundredweight = 112 lb cwt 50.8024 kg 0.0196841 1 rod (perch, pole) rd 5.0292 m 0.198839 1 long hundredweight l cwt 50.8024 kg 0.0196841 1 chain = 100 links ch 20.1168 m 0.0497097 1 short hundredweight sh cwt 45.3592 kg 0.0220462 1 furlong = 220 yd fur 0.201168 km 4.97097 1 ton = 1 long ton tn, l tn 1.016047 t 0.984206 1 mile (Land Mile) mi 1.60934 km 0.62137 1 short ton = 2000 lb sh tn 0.907185 t 1.102311 1 nautical mile (intl.) n mi, NM 1.852 km 0.539957 1 knot (Knoten) kn 1.852 km/h 0.539957 UNITS OF FORCE 1 pound-weight lb wt 4.448221 N 0.2248089 UNITS OF AREA 1 pound-force LB, lbf 4.448221 N 0.2248089 1 square inch sq in 6.4516 cm2 0.155000 1 poundal pdl 0.138255 N 7.23301 1 circular inch 5.0671 cm2 0.197352 1 kilogram-force kgf, kgp 9.80665 N 0.1019716 1 square foot = 144 sq in sq ft 929.03 cm2 1.0764 x 10-4 1 short ton-weight sh tn wt 8.896444 kN 0.1124045 1 square yard = 9 sq ft sq yd 0.83613 m2 1.19599 1 long ton-weight l tn wt 9.964015 kN 0.1003611 1 acre = 4 roods 4046.8 -

What Is a Rain Barrel?
What is a rain barrel? A rain barrel is a container used to collect and store rainwater from your roof that would otherwise be lost to runoff and diverted out onto your property or to a storm drain and eventually to local streams or rivers. Rain barrels are also an economical way to store rain water to be used as a secondary water supply for indoor plants, flower gardens, lawns, fill the bird bath, and washing cars and windows. Rain barrels are usually about 40-60 gallons and can be purchased or made relatively easily. The parts are available at any hardware store. *Stored water is not used for drinking or bathing* Why use rain barrels? Every time it rains, unabsorbed water rushes to storm drains and directly into our local waterways. Often times this runoff carries with it pollutants it has picked up along the way depositing in them into local waterways. Any rainwater in an urban or suburban area that does not evaporate or infiltrate into the ground is considered stormwater. Infiltration is when water on the ground surface soaks into the soil. Impervious surfaces like roofs, asphalt, and concrete do not allow Rain water from your roof and driveway travels to the street and into storm drains for the infiltration to occur. eventually draining into our creeks, lakes, and rivers. Infiltration of water on pervious surfaces is important because it reduces the amount runoff and the possibility of erosion and pollutants leaving a site and entering a waterway. What can rain barrels do for you? Healthier plants. -

Calculating Board Feet Board Feet Linear Feet Z "Board Feet" Is a Measurement of Lumber Square Feet Volume
Calculating Board Feet board Feet linear feet z "Board Feet" is a measurement of lumber square feet volume. z A board foot is equal to 144 cubic inches of wood. TED 126 Spring 2007 z Actually it's easy to calculate using the following formula: Bd. Ft. = T (inches) x W (inches) x L (feet) / 12 2 Board Feet Board Feet z When you are figuring up board feet, keep in mind a waste factor. Bd. Ft. = T (inches) x W (inches) x L (feet) / 12 z If you purchase good clear material add about 15% for waste, Bd. Ft. = T (inches) x W (inches) x L (inches) / 144 z if you elect to use lower grade material you will have to allow for defects and more wasted material ---add about 30%. 3 4 Board Feet and Linear feet Board Feet and Linear feet z A linear foot is a measure of length 12 inches z To convert linear feet to board feet: long and a Thickness” x Width” x Length’ ÷ 12 z board foot is a number calculated by determining the volume of a board that is 12 z To convert board feet to linear feet: inches wide and 1 inch thick. • In other words, a 1" x 6" board that measures 24" 12 ÷ Thickness” x Width” x Board Foot long is exactly one board foot. (width" x thickness" x length' / 12) 5 6 1 Linear feet and Square Feet The math…. z It is not possible to convert linear footage into z A Linear Feet is just a measurement of square footage because a linear foot is only one length and does not take into account its dimension and a square foot is two dimensions, width or thickness. -

Feasibility Analysis of a Small Log Sawmill in Southeast Alaska
1 Feasibility analysis of a small log sawmill in Southeast Alaska Executive Summary Unlike most Southern Yellow Pine sawmills, a small log mill in Southeast Alaska that manufactured lumber from 60-year old trees, would not be profitable. There are many additional operating costs in the remote forests of Southeast Alaska that the Southern Yellow Pine sawmills do not incur. The two most costly disadvantages are; 1. An additional $SO per thousand board feet of lumber that is required to transport lumber from Southeast Alaska to the Pacific Northwest and, 2. The lack ofany reasonable economy of scale. The small timber sale volume projected to be available to the manufacturing industry in Southeast Alaska is inadequate to support more than a single mid-size sawmill. Consequently the regions sawmills will not produce any income from the residual products - chips, sawdust and bark. The chips are currently barged to pulp mills in the Pacific Northwest and Canada, while the Yellow Pine sawmills can deliver chips to nearby pulp mills at a much lower cost than the Alaska sawmills. Similarly, there are no fiberboard plants to utilize the sawdust from Southeast Alaska sawmills and there is no market for the bark in Southeast Alaska. Instead, most ofthe sawdust and bark must be disposed of in landfills. Introduction The purpose of this analysis is to compare several financial estimates of the feasibility of manufacturing lumber from immature young growth timber in Southeast Alaska. Four sawmill proformas are 1 examined : 1. A summary of five actual Southern Yellow Pine sawmills. This proforma was used because much of the rhetoric surrounding the Secretary of Agriculture unilateral decision to transition to 60+ year old Alaska young growth was based on assertions that Yellow Pine sawmills harvest their 2 timber before age 60 • Other than the obvious difference in tree species, the yellow pine region has much different logistic issues than Southeast Alaska. -

2.1 Definition of the SI
CCPR/16-53 Modifications to the Draft of the ninth SI Brochure dated 16 September 2016 recommended by the CCPR to the CCU via the CCPR president Takashi Usuda, Wednesday 14 December 2016. The text in black is a selection of paragraphs from the brochure with the section title for indication. The sentences to be modified appear in red. 2.1 Definition of the SI Like for any value of a quantity, the value of a fundamental constant can be expressed as the product of a number and a unit as Q = {Q} [Q]. The definitions below specify the exact numerical value of each constant when its value is expressed in the corresponding SI unit. By fixing the exact numerical value the unit becomes defined, since the product of the numerical value {Q} and the unit [Q] has to equal the value Q of the constant, which is postulated to be invariant. The seven constants are chosen in such a way that any unit of the SI can be written either through a defining constant itself or through products or ratios of defining constants. The International System of Units, the SI, is the system of units in which the unperturbed ground state hyperfine splitting frequency of the caesium 133 atom Cs is 9 192 631 770 Hz, the speed of light in vacuum c is 299 792 458 m/s, the Planck constant h is 6.626 070 040 ×1034 J s, the elementary charge e is 1.602 176 620 8 ×1019 C, the Boltzmann constant k is 1.380 648 52 ×1023 J/K, 23 -1 the Avogadro constant NA is 6.022 140 857 ×10 mol , 12 the luminous efficacy of monochromatic radiation of frequency 540 ×10 hertz Kcd is 683 lm/W.