Appendix A: Symbols and Prefixes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

American and BRITISH UNITS of Measurement to SI UNITS
AMERICAN AND BRITISH UNITS OF MEASUREMENT TO SI UNITS UNIT & ABBREVIATION SI UNITS CONVERSION* UNIT & ABBREVIATION SI UNITS CONVERSION* UNITS OF LENGTH UNITS OF MASS 1 inch = 40 lines in 2.54 cm 0.393701 1 grain gr 64.7989 mg 0.0154324 1 mil 25.4 µm 0.03937 1 dram dr 1.77185 g 0.564383 1 line 0.635 mm 1.57480 1 ounce = 16 drams oz 28.3495 g 0.0352739 1 foot = 12 in = 3 hands ft 30.48 cm 0.0328084 1 pound = 16 oz lb 0.453592 kg 2.204622 1 yard = 3 feet = 4 spans yd 0.9144 m 1.09361 1 quarter = 28 lb 12.7006 kg 0.078737 1 fathom = 2 yd fath 1.8288 m 0.546807 1 hundredweight = 112 lb cwt 50.8024 kg 0.0196841 1 rod (perch, pole) rd 5.0292 m 0.198839 1 long hundredweight l cwt 50.8024 kg 0.0196841 1 chain = 100 links ch 20.1168 m 0.0497097 1 short hundredweight sh cwt 45.3592 kg 0.0220462 1 furlong = 220 yd fur 0.201168 km 4.97097 1 ton = 1 long ton tn, l tn 1.016047 t 0.984206 1 mile (Land Mile) mi 1.60934 km 0.62137 1 short ton = 2000 lb sh tn 0.907185 t 1.102311 1 nautical mile (intl.) n mi, NM 1.852 km 0.539957 1 knot (Knoten) kn 1.852 km/h 0.539957 UNITS OF FORCE 1 pound-weight lb wt 4.448221 N 0.2248089 UNITS OF AREA 1 pound-force LB, lbf 4.448221 N 0.2248089 1 square inch sq in 6.4516 cm2 0.155000 1 poundal pdl 0.138255 N 7.23301 1 circular inch 5.0671 cm2 0.197352 1 kilogram-force kgf, kgp 9.80665 N 0.1019716 1 square foot = 144 sq in sq ft 929.03 cm2 1.0764 x 10-4 1 short ton-weight sh tn wt 8.896444 kN 0.1124045 1 square yard = 9 sq ft sq yd 0.83613 m2 1.19599 1 long ton-weight l tn wt 9.964015 kN 0.1003611 1 acre = 4 roods 4046.8 -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

SI and CGS Units in Electromagnetism
SI and CGS Units in Electromagnetism Jim Napolitano January 7, 2010 These notes are meant to accompany the course Electromagnetic Theory for the Spring 2010 term at RPI. The course will use CGS units, as does our textbook Classical Electro- dynamics, 2nd Ed. by Hans Ohanian. Up to this point, however, most students have used the International System of Units (SI, also known as MKSA) for mechanics, electricity and magnetism. (I believe it is easy to argue that CGS is more appropriate for teaching elec- tromagnetism to physics students.) These notes are meant to smooth the transition, and to augment the discussion in Appendix 2 of your textbook. The base units1 for mechanics in SI are the meter, kilogram, and second (i.e. \MKS") whereas in CGS they are the centimeter, gram, and second. Conversion between these base units and all the derived units are quite simply given by an appropriate power of 10. For electromagnetism, SI adds a new base unit, the Ampere (\A"). This leads to a world of complications when converting between SI and CGS. Many of these complications are philosophical, but this note does not discuss such issues. For a good, if a bit flippant, on- line discussion see http://info.ee.surrey.ac.uk/Workshop/advice/coils/unit systems/; for a more scholarly article, see \On Electric and Magnetic Units and Dimensions", by R. T. Birge, The American Physics Teacher, 2(1934)41. Electromagnetism: A Preview Electricity and magnetism are not separate phenomena. They are different manifestations of the same phenomenon, called electromagnetism. One requires the application of special relativity to see how electricity and magnetism are united. -
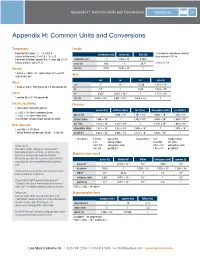
Appendix H: Common Units and Conversions Appendices 215
Appendix H: Common Units and Conversions Appendices 215 Appendix H: Common Units and Conversions Temperature Length Fahrenheit to Celsius: °C = (°F-32)/1.8 1 micrometer (sometimes referred centimeter (cm) meter (m) inch (in) Celsius to Fahrenheit: °F = (1.8 × °C) + 32 to as micron) = 10-6 m Fahrenheit to Kelvin: convert °F to °C, then add 273.15 centimeter (cm) 1 1.000 × 10–2 0.3937 -3 Celsius to Kelvin: add 273.15 meter (m) 100 1 39.37 1 mil = 10 in Volume inch (in) 2.540 2.540 × 10–2 1 –3 3 1 liter (l) = 1.000 × 10 cubic meters (m ) = 61.02 Area cubic inches (in3) cm2 m2 in2 circ mil Mass cm2 1 10–4 0.1550 1.974 × 105 1 kilogram (kg) = 1000 grams (g) = 2.205 pounds (lb) m2 104 1 1550 1.974 × 109 Force in2 6.452 6.452 × 10–4 1 1.273 × 106 1 newton (N) = 0.2248 pounds (lb) circ mil 5.067 × 10–6 5.067 × 10–10 7.854 × 10–7 1 Electric resistivity Pressure 1 micro-ohm-centimeter (µΩ·cm) pascal (Pa) millibar (mbar) torr (Torr) atmosphere (atm) psi (lbf/in2) = 1.000 × 10–6 ohm-centimeter (Ω·cm) –2 –3 –6 –4 = 1.000 × 10–8 ohm-meter (Ω·m) pascal (Pa) 1 1.000 × 10 7.501 × 10 9.868 × 10 1.450 × 10 = 6.015 ohm-circular mil per foot (Ω·circ mil/ft) millibar (mbar) 1.000 × 102 1 7.502 × 10–1 9.868 × 10–4 1.450 × 10–2 2 0 –3 –2 Heat flow rate torr (Torr) 1.333 × 10 1.333 × 10 1 1.316 × 10 1.934 × 10 5 3 2 1 1 watt (W) = 3.413 Btu/h atmosphere (atm) 1.013 × 10 1.013 × 10 7.600 × 10 1 1.470 × 10 1 British thermal unit per hour (Btu/h) = 0.2930 W psi (lbf/in2) 6.897 × 103 6.895 × 101 5.172 × 101 6.850 × 10–2 1 1 torr (Torr) = 133.332 pascal (Pa) 1 pascal (Pa) = 0.01 millibar (mbar) 1.33 millibar (mbar) 0.007501 torr (Torr) –6 A Note on SI 0.001316 atmosphere (atm) 9.87 × 10 atmosphere (atm) 2 –4 2 The values in this catalog are expressed in 0.01934 psi (lbf/in ) 1.45 × 10 psi (lbf/in ) International System of Units, or SI (from the Magnetic induction B French Le Système International d’Unités). -

Paper Code and Title: H01RS Residential Space Designing Module Code and Name: H01RS19 Light – Measurement, Related Terms and Units Name of the Content Writer: Dr
Paper Code and Title: H01RS Residential Space Designing Module Code and Name: H01RS19 Light – measurement, related terms and units Name of the Content Writer: Dr. S. Visalakshi Rajeswari LIGHT – MEASUREMENT, RELATED TERMS AND UNITS Introduction Light is that part of the electromagnetic spectrum which will stimulate a response in the receptors of the eye. Its frequency usually expressed as wavelength determines the colour of light and its amplitude determines its intensity. Accommodation from the individual’s part enables focusing of vision. Hence the need to study lighting in interiors. Especially when activities are carried out indoors it is necessary to provide some sort of artificial illumination. In such circumstances, the designer should be aware of what (lighting) is provided and the satisfaction the ‘user’ derives out of it. 1. The radiant energy spectrum Vs visible spectrum Light is visually evaluated radiant energy (electromagnetic), which moves at a constant speed in vacuum. The entire radiant energy spectrum consists of waves of radiant energy that vary in wavelength of a wide range; an array of all rays - cosmic, gamma, UV, infra red, radar, x rays, the visible spectrum, FM, TV- and radio broadcast waves and power transmission. The portion of the radiant energy which is seen as light, identified as the spectrum visible to the human eye ranges from about 380 (400) to 780 (700) mµ (referred to as nanometers or millimicrons). A nanometer (nm) is a unit of wavelength equal to 10 -9 m. Light can thus be thought of as the aspect of radiant energy that is visible. Colour perception is attributed to the varying wavelengths noticeable within the spectrum of visible light. -

The International System of Units (SI)
NAT'L INST. OF STAND & TECH NIST National Institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 330 2001 Edition The International System of Units (SI) 4. Barry N. Taylor, Editor r A o o L57 330 2oOI rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -

Physics, Chapter 30: Magnetic Fields of Currents
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Robert Katz Publications Research Papers in Physics and Astronomy 1-1958 Physics, Chapter 30: Magnetic Fields of Currents Henry Semat City College of New York Robert Katz University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/physicskatz Part of the Physics Commons Semat, Henry and Katz, Robert, "Physics, Chapter 30: Magnetic Fields of Currents" (1958). Robert Katz Publications. 151. https://digitalcommons.unl.edu/physicskatz/151 This Article is brought to you for free and open access by the Research Papers in Physics and Astronomy at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Robert Katz Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 30 Magnetic Fields of Currents 30-1 Magnetic Field around an Electric Current The first evidence for the existence of a magnetic field around an electric current was observed in 1820 by Hans Christian Oersted (1777-1851). He found that a wire carrying current caused a freely pivoted compass needle B D N rDirection I of current D II. • ~ I I In wire ' \ N I I c , I s c (a) (b) Fig. 30-1 Oersted's experiment. Compass needle is deflected toward the west when the wire CD carrying current is placed above it and the direction of the current is toward the north, from C to D. in its vicinity to be deflected. If the current in a long straight wire is directed from C to D, as shown in Figure 30-1, a compass needle below it, whose initial orientation is shown in dotted lines, will have its north pole deflected to the left and its south pole deflected to the right. -

Comparing Many-Body Approaches Against the Helium Atom Exact Solution
SciPost Phys. 6, 040 (2019) Comparing many-body approaches against the helium atom exact solution Jing Li1,2, N. D. Drummond3, Peter Schuck1,4,5 and Valerio Olevano1,2,6? 1 Université Grenoble Alpes, 38000 Grenoble, France 2 CNRS, Institut Néel, 38042 Grenoble, France 3 Department of Physics, Lancaster University, Lancaster LA1 4YB, United Kingdom 4 CNRS, LPMMC, 38042 Grenoble, France 5 CNRS, Institut de Physique Nucléaire, IN2P3, Université Paris-Sud, 91406 Orsay, France 6 European Theoretical Spectroscopy Facility (ETSF) ? [email protected] Abstract Over time, many different theories and approaches have been developed to tackle the many-body problem in quantum chemistry, condensed-matter physics, and nuclear physics. Here we use the helium atom, a real system rather than a model, and we use the exact solution of its Schrödinger equation as a benchmark for comparison between methods. We present new results beyond the random-phase approximation (RPA) from a renormalized RPA (r-RPA) in the framework of the self-consistent RPA (SCRPA) originally developed in nuclear physics, and compare them with various other approaches like configuration interaction (CI), quantum Monte Carlo (QMC), time-dependent density- functional theory (TDDFT), and the Bethe-Salpeter equation on top of the GW approx- imation. Most of the calculations are consistently done on the same footing, e.g. using the same basis set, in an effort for a most faithful comparison between methods. Copyright J. Li et al. Received 15-01-2019 This work is licensed under the Creative Commons Accepted 21-03-2019 Check for Attribution 4.0 International License. -

Introduction to Numerical Projects Format for Electronic Delivery Of
Introduction to numerical projects Here follows a brief recipe and recommendation on how to write a report for each project. • Give a short description of the nature of the problem and the eventual numerical methods you have used. • Describe the algorithm you have used and/or developed. Here you may find it con- venient to use pseudocoding. In many cases you can describe the algorithm in the program itself. • Include the source code of your program. Comment your program properly. • If possible, try to find analytic solutions, or known limits in order to test your program when developing the code. • Include your results either in figure form or in a table. Remember to label your results. All tables and figures should have relevant captions and labels on the axes. • Try to evaluate the reliabilty and numerical stability/precision of your results. If pos- sible, include a qualitative and/or quantitative discussion of the numerical stability, eventual loss of precision etc. • Try to give an interpretation of you results in your answers to the problems. • Critique: if possible include your comments and reflections about the exercise, whether you felt you learnt something, ideas for improvements and other thoughts you’ve made when solving the exercise. We wish to keep this course at the interactive level and your comments can help us improve it. We do appreciate your comments. • Try to establish a practice where you log your work at the computerlab. You may find such a logbook very handy at later stages in your work, especially when you don’t properly remember what a previous test version of your program did. -

CAR-ANS PART 05 Issue No. 2 Units of Measurement to Be Used In
CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES Part 5 Governing UNITS OF MEASUREMENT TO BE USED IN AIR AND GROUND OPERATIONS CIVIL AVIATION AUTHORITY OF THE PHILIPPINES Old MIA Road, Pasay City1301 Metro Manila INTENTIONALLY LEFT BLANK CAR-ANS PART 5 Republic of the Philippines CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES (CAR-ANS) Part 5 UNITS OF MEASUREMENTS TO BE USED IN AIR AND GROUND OPERATIONS 22 APRIL 2016 EFFECTIVITY Part 5 of the Civil Aviation Regulations-Air Navigation Services are issued under the authority of Republic Act 9497 and shall take effect upon approval of the Board of Directors of the CAAP. APPROVED BY: LT GEN WILLIAM K HOTCHKISS III AFP (RET) DATE Director General Civil Aviation Authority of the Philippines Issue 2 15-i 16 May 2016 CAR-ANS PART 5 FOREWORD This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was formulated and issued by the Civil Aviation Authority of the Philippines (CAAP), prescribing the standards and recommended practices for units of measurements to be used in air and ground operations within the territory of the Republic of the Philippines. This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was developed based on the Standards and Recommended Practices prescribed by the International Civil Aviation Organization (ICAO) as contained in Annex 5 which was first adopted by the council on 16 April 1948 pursuant to the provisions of Article 37 of the Convention of International Civil Aviation (Chicago 1944), and consequently became applicable on 1 January 1949. The provisions contained herein are issued by authority of the Director General of the Civil Aviation Authority of the Philippines and will be complied with by all concerned. -

Radiometric and Photometric Measurements with TAOS Photosensors Contributed by Todd Bishop March 12, 2007 Valid
TAOS Inc. is now ams AG The technical content of this TAOS application note is still valid. Contact information: Headquarters: ams AG Tobelbaderstrasse 30 8141 Unterpremstaetten, Austria Tel: +43 (0) 3136 500 0 e-Mail: [email protected] Please visit our website at www.ams.com NUMBER 21 INTELLIGENT OPTO SENSOR DESIGNER’S NOTEBOOK Radiometric and Photometric Measurements with TAOS PhotoSensors contributed by Todd Bishop March 12, 2007 valid ABSTRACT Light Sensing applications use two measurement systems; Radiometric and Photometric. Radiometric measurements deal with light as a power level, while Photometric measurements deal with light as it is interpreted by the human eye. Both systems of measurement have units that are parallel to each other, but are useful for different applications. This paper will discuss the differencesstill and how they can be measured. AG RADIOMETRIC QUANTITIES Radiometry is the measurement of electromagnetic energy in the range of wavelengths between ~10nm and ~1mm. These regions are commonly called the ultraviolet, the visible and the infrared. Radiometry deals with light (radiant energy) in terms of optical power. Key quantities from a light detection point of view are radiant energy, radiant flux and irradiance. SI Radiometryams Units Quantity Symbol SI unit Abbr. Notes Radiant energy Q joule contentJ energy radiant energy per Radiant flux Φ watt W unit time watt per power incident on a Irradiance E square meter W·m−2 surface Energy is an SI derived unit measured in joules (J). The recommended symbol for energy is Q. Power (radiant flux) is another SI derived unit. It is the derivative of energy with respect to time, dQ/dt, and the unit is the watt (W). -

Photoelectric Effect Prac
Name ___________________. Photoelectric Effect Prac 1. The threshold frequency in a photoelectric experiment is most closely related to the A) brightness of the incident light B) thickness of the photoemissive metal C) area of the photoemissive metal D) work function of the photoemissive metal 2. When a source of dim orange light shines on a photosensitive metal, no photoelectrons are ejected from its surface. What could be done to increase the likelihood of producing photoelectrons? A) Replace the orange light source with a red light source. B) Replace the orange light source with a higher frequency light source. C) Increase the brightness of the orange light source. D) Increase the angle at which the photons of orange light strike the metal. 3. The threshold frequency for a photoemissive surface is hertz. Which color light, if incident upon the surface, may produce photoelectrons? A) blue B) green C) yellow D) red 4. The threshold frequency of a metal surface is in the violet light region. What type of radiation will cause photoelectrons to be emitted from the metal's surface? A) infrared light B) red light C) ultraviolet light D) radio waves 5. Photons with an energy of 7.9 electron-volts strike a zinc plate, causing the emission of photoelectrons with a maximum kinetic energy of 4.0 electronvolts. The work function of the zinc plate is A) 11.9 eV B) 7.9 eV C) 3.9 eV D) 4.0 eV 6. The threshold frequency for a photoemissive surface is 1.0 × 1014 hertz. What is the work function of the surface? A) 1.0 × 10–14 J B) 6.6 × 10–20 J C) 6.6 × 10–48 J D) 2.2 × 10–28 J 7.