Radiometry and Photometry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -

Appendix A: Symbols and Prefixes
Appendix A: Symbols and Prefixes (Appendix A last revised November 2020) This appendix of the Author's Kit provides recommendations on prefixes, unit symbols and abbreviations, and factors for conversion into units of the International System. Prefixes Recommended prefixes indicating decimal multiples or submultiples of units and their symbols are as follows: Multiple Prefix Abbreviation 1024 yotta Y 1021 zetta Z 1018 exa E 1015 peta P 1012 tera T 109 giga G 106 mega M 103 kilo k 102 hecto h 10 deka da 10-1 deci d 10-2 centi c 10-3 milli m 10-6 micro μ 10-9 nano n 10-12 pico p 10-15 femto f 10-18 atto a 10-21 zepto z 10-24 yocto y Avoid using compound prefixes, such as micromicro for pico and kilomega for giga. The abbreviation of a prefix is considered to be combined with the abbreviation/symbol to which it is directly attached, forming with it a new unit symbol, which can be raised to a positive or negative power and which can be combined with other unit abbreviations/symbols to form abbreviations/symbols for compound units. For example: 1 cm3 = (10-2 m)3 = 10-6 m3 1 μs-1 = (10-6 s)-1 = 106 s-1 1 mm2/s = (10-3 m)2/s = 10-6 m2/s Abbreviations and Symbols Whenever possible, avoid using abbreviations and symbols in paragraph text; however, when it is deemed necessary to use such, define all but the most common at first use. The following is a recommended list of abbreviations/symbols for some important units. -

Luminance Requirements for Lighted Signage
Luminance Requirements for Lighted Signage Jean Paul Freyssinier, Nadarajah Narendran, John D. Bullough Lighting Research Center Rensselaer Polytechnic Institute, Troy, NY 12180 www.lrc.rpi.edu Freyssinier, J.P., N. Narendran, and J.D. Bullough. 2006. Luminance requirements for lighted signage. Sixth International Conference on Solid State Lighting, Proceedings of SPIE 6337, 63371M. Copyright 2006 Society of Photo-Optical Instrumentation Engineers. This paper was published in the Sixth International Conference on Solid State Lighting, Proceedings of SPIE and is made available as an electronic preprint with permission of SPIE. One print or electronic copy may be made for personal use only. Systematic or multiple reproduction, distribution to multiple locations via electronic or other means, duplication of any material in this paper for a fee or for commercial purposes, or modification of the content of the paper are prohibited. Luminance Requirements for Lighted Signage Jean Paul Freyssinier*, Nadarajah Narendran, John D. Bullough Lighting Research Center, Rensselaer Polytechnic Institute, 21 Union Street, Troy, NY 12180 USA ABSTRACT Light-emitting diode (LED) technology is presently targeted to displace traditional light sources in backlighted signage. The literature shows that brightness and contrast are perhaps the two most important elements of a sign that determine its attention-getting capabilities and its legibility. Presently, there are no luminance standards for signage, and the practice of developing brighter signs to compete with signs in adjacent businesses is becoming more commonplace. Sign luminances in such cases may far exceed what people usually need for identifying and reading a sign. Furthermore, the practice of higher sign luminance than needed has many negative consequences, including higher energy use and light pollution. -

Report on Digital Sign Brightness
REPORT ON DIGITAL SIGN BRIGHTNESS Prepared for the Nevada State Department of Transportation, Washoe County, City of Reno and City of Sparks By Jerry Wachtel, President, The Veridian Group, Inc., Berkeley, CA November 2014 1 Table of Contents PART 1 ......................................................................................................................... 3 Introduction. ................................................................................................................ 3 Background. ................................................................................................................. 3 Key Terms and Definitions. .......................................................................................... 4 Luminance. .................................................................................................................................................................... 4 Illuminance. .................................................................................................................................................................. 5 Reflected Light vs. Emitted Light (Traditional Signs vs. Electronic Signs). ...................................... 5 Measuring Luminance and Illuminance ........................................................................ 6 Measuring Luminance. ............................................................................................................................................. 6 Measuring Illuminance. .......................................................................................................................................... -

Lecture 3: the Sensor
4.430 Daylighting Human Eye ‘HDR the old fashioned way’ (Niemasz) Massachusetts Institute of Technology ChriChristoph RstophReeiinhartnhart Department of Architecture 4.4.430 The430The SeSensnsoror Building Technology Program Happy Valentine’s Day Sun Shining on a Praline Box on February 14th at 9.30 AM in Boston. 1 Happy Valentine’s Day Falsecolor luminance map Light and Human Vision 2 Human Eye Outside view of a human eye Ophtalmogram of a human retina Retina has three types of photoreceptors: Cones, Rods and Ganglion Cells Day and Night Vision Photopic (DaytimeVision): The cones of the eye are of three different types representing the three primary colors, red, green and blue (>3 cd/m2). Scotopic (Night Vision): The rods are repsonsible for night and peripheral vision (< 0.001 cd/m2). Mesopic (Dim Light Vision): occurs when the light levels are low but one can still see color (between 0.001 and 3 cd/m2). 3 VisibleRange Daylighting Hanbook (Reinhart) The human eye can see across twelve orders of magnitude. We can adapt to about 10 orders of magnitude at a time via the iris. Larger ranges take time and require ‘neural adaptation’. Transition Spaces Outside Atrium Circulation Area Final destination 4 Luminous Response Curve of the Human Eye What is daylight? Daylight is the visible part of the electromagnetic spectrum that lies between 380 and 780 nm. UV blue green yellow orange red IR 380 450 500 550 600 650 700 750 wave length (nm) 5 Photometric Quantities Characterize how a space is perceived. Illuminance Luminous Flux Luminance Luminous Intensity Luminous Intensity [Candela] ~ 1 candela Courtesy of Matthew Bowden at www.digitallyrefreshing.com. -
Plant Lighting Basics and Applications
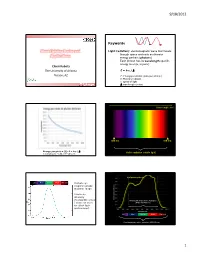
9/18/2012 Keywords Plant Lighting Basics and Light (radiation): electromagnetic wave that travels through space and exits as discrete Applications energy packets (photons) Each photon has its wavelength-specific energy level (E, in joule) Chieri Kubota The University of Arizona E = h·c / Tucson, AZ E: Energy per photon (joule per photon) h: Planck’s constant c: speed of light Greensys 2011, Greece : wavelength (meter) Wavelength (nm) 380 nm 780 nm Energy per photon [J]: E = h·c / Visible radiation (visible light) mole photons = 6.02 x 1023 photons Leaf photosynthesis UV Blue Green Red Far red Human eye response peaks at green range. Luminous intensity (footcandle or lux Photosynthetically Active Radiation ) does not work (PAR, 400-700 nm) for plant light environment. UV Blue Green Red Far red Plant biologically active radiation (300-800 nm) 1 9/18/2012 Chlorophyll a Phytochrome response Chlorophyll b UV Blue Green Red Far red Absorption spectra of isolated chlorophyll Absorptance 400 500 600 700 Wavelength (nm) Daily light integral (DLI or Daily Light Unit and Terminology PPF) Radiation Photons Visible light •Total amount of photosynthetically active “Base” unit Energy (J) Photons (mol) Luminous intensity (cd) radiation (400‐700 nm) received per sq Flux [total amount received Radiant flux Photon flux Luminous flux meter per day or emitted per time] (J s-1) or (W) (mol s-1) (lm) •Unit: mole per sq meter per day (mol m‐2 d‐1) Flux density [total amount Radiant flux density Photon flux (density) Illuminance, • Under optimal conditions, plant growth is received per area per time] (W m-2) (mol m-2 s-1) Luminous flux density highly correlated with DLI. -

Light and Illumination
ChapterChapter 3333 -- LightLight andand IlluminationIllumination AAA PowerPointPowerPointPowerPoint PresentationPresentationPresentation bybyby PaulPaulPaul E.E.E. Tippens,Tippens,Tippens, ProfessorProfessorProfessor ofofof PhysicsPhysicsPhysics SouthernSouthernSouthern PolytechnicPolytechnicPolytechnic StateStateState UniversityUniversityUniversity © 2007 Objectives:Objectives: AfterAfter completingcompleting thisthis module,module, youyou shouldshould bebe ableable to:to: •• DefineDefine lightlight,, discussdiscuss itsits properties,properties, andand givegive thethe rangerange ofof wavelengthswavelengths forfor visiblevisible spectrum.spectrum. •• ApplyApply thethe relationshiprelationship betweenbetween frequenciesfrequencies andand wavelengthswavelengths forfor opticaloptical waves.waves. •• DefineDefine andand applyapply thethe conceptsconcepts ofof luminousluminous fluxflux,, luminousluminous intensityintensity,, andand illuminationillumination.. •• SolveSolve problemsproblems similarsimilar toto thosethose presentedpresented inin thisthis module.module. AA BeginningBeginning DefinitionDefinition AllAll objectsobjects areare emittingemitting andand absorbingabsorbing EMEM radiaradia-- tiontion.. ConsiderConsider aa pokerpoker placedplaced inin aa fire.fire. AsAs heatingheating occurs,occurs, thethe 1 emittedemitted EMEM waveswaves havehave 2 higherhigher energyenergy andand 3 eventuallyeventually becomebecome visible.visible. 4 FirstFirst redred .. .. .. thenthen white.white. LightLightLight maymaymay bebebe defineddefineddefined -

Applied Spectroscopy Spectroscopic Nomenclature
Applied Spectroscopy Spectroscopic Nomenclature Absorbance, A Negative logarithm to the base 10 of the transmittance: A = –log10(T). (Not used: absorbancy, extinction, or optical density). (See Note 3). Absorptance, α Ratio of the radiant power absorbed by the sample to the incident radiant power; approximately equal to (1 – T). (See Notes 2 and 3). Absorption The absorption of electromagnetic radiation when light is transmitted through a medium; hence ‘‘absorption spectrum’’ or ‘‘absorption band’’. (Not used: ‘‘absorbance mode’’ or ‘‘absorbance band’’ or ‘‘absorbance spectrum’’ unless the ordinate axis of the spectrum is Absorbance.) (See Note 3). Absorption index, k See imaginary refractive index. Absorptivity, α Internal absorbance divided by the product of sample path length, ℓ , and mass concentration, ρ , of the absorbing material. A / α = i ρℓ SI unit: m2 kg–1. Common unit: cm2 g–1; L g–1 cm–1. (Not used: absorbancy index, extinction coefficient, or specific extinction.) Attenuated total reflection, ATR A sampling technique in which the evanescent wave of a beam that has been internally reflected from the internal surface of a material of high refractive index at an angle greater than the critical angle is absorbed by a sample that is held very close to the surface. (See Note 3.) Attenuation The loss of electromagnetic radiation caused by both absorption and scattering. Beer–Lambert law Absorptivity of a substance is constant with respect to changes in path length and concentration of the absorber. Often called Beer’s law when only changes in concentration are of interest. Brewster’s angle, θB The angle of incidence at which the reflection of p-polarized radiation is zero. -

Section 22-3: Energy, Momentum and Radiation Pressure
Answer to Essential Question 22.2: (a) To find the wavelength, we can combine the equation with the fact that the speed of light in air is 3.00 " 108 m/s. Thus, a frequency of 1 " 1018 Hz corresponds to a wavelength of 3 " 10-10 m, while a frequency of 90.9 MHz corresponds to a wavelength of 3.30 m. (b) Using Equation 22.2, with c = 3.00 " 108 m/s, gives an amplitude of . 22-3 Energy, Momentum and Radiation Pressure All waves carry energy, and electromagnetic waves are no exception. We often characterize the energy carried by a wave in terms of its intensity, which is the power per unit area. At a particular point in space that the wave is moving past, the intensity varies as the electric and magnetic fields at the point oscillate. It is generally most useful to focus on the average intensity, which is given by: . (Eq. 22.3: The average intensity in an EM wave) Note that Equations 22.2 and 22.3 can be combined, so the average intensity can be calculated using only the amplitude of the electric field or only the amplitude of the magnetic field. Momentum and radiation pressure As we will discuss later in the book, there is no mass associated with light, or with any EM wave. Despite this, an electromagnetic wave carries momentum. The momentum of an EM wave is the energy carried by the wave divided by the speed of light. If an EM wave is absorbed by an object, or it reflects from an object, the wave will transfer momentum to the object. -

CNT-Based Solar Thermal Coatings: Absorptance Vs. Emittance
coatings Article CNT-Based Solar Thermal Coatings: Absorptance vs. Emittance Yelena Vinetsky, Jyothi Jambu, Daniel Mandler * and Shlomo Magdassi * Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 9190401, Israel; [email protected] (Y.V.); [email protected] (J.J.) * Correspondence: [email protected] (D.M.); [email protected] (S.M.) Received: 15 October 2020; Accepted: 13 November 2020; Published: 17 November 2020 Abstract: A novel approach for fabricating selective absorbing coatings based on carbon nanotubes (CNTs) for mid-temperature solar–thermal application is presented. The developed formulations are dispersions of CNTs in water or solvents. Being coated on stainless steel (SS) by spraying, these formulations provide good characteristics of solar absorptance. The effect of CNT concentration and the type of the binder and its ratios to the CNT were investigated. Coatings based on water dispersions give higher adsorption, but solvent-based coatings enable achieving lower emittance. Interestingly, the binder was found to be responsible for the high emittance, yet, it is essential for obtaining good adhesion to the SS substrate. The best performance of the coatings requires adjusting the concentration of the CNTs and their ratio to the binder to obtain the highest absorptance with excellent adhesion; high absorptance is obtained at high CNT concentration, while good adhesion requires a minimum ratio between the binder/CNT; however, increasing the binder concentration increases the emissivity. The best coatings have an absorptance of ca. 90% with an emittance of ca. 0.3 and excellent adhesion to stainless steel. Keywords: carbon nanotubes (CNTs); binder; dispersion; solar thermal coating; absorptance; emittance; adhesion; selectivity 1. -

2.1 Definition of the SI
CCPR/16-53 Modifications to the Draft of the ninth SI Brochure dated 16 September 2016 recommended by the CCPR to the CCU via the CCPR president Takashi Usuda, Wednesday 14 December 2016. The text in black is a selection of paragraphs from the brochure with the section title for indication. The sentences to be modified appear in red. 2.1 Definition of the SI Like for any value of a quantity, the value of a fundamental constant can be expressed as the product of a number and a unit as Q = {Q} [Q]. The definitions below specify the exact numerical value of each constant when its value is expressed in the corresponding SI unit. By fixing the exact numerical value the unit becomes defined, since the product of the numerical value {Q} and the unit [Q] has to equal the value Q of the constant, which is postulated to be invariant. The seven constants are chosen in such a way that any unit of the SI can be written either through a defining constant itself or through products or ratios of defining constants. The International System of Units, the SI, is the system of units in which the unperturbed ground state hyperfine splitting frequency of the caesium 133 atom Cs is 9 192 631 770 Hz, the speed of light in vacuum c is 299 792 458 m/s, the Planck constant h is 6.626 070 040 ×1034 J s, the elementary charge e is 1.602 176 620 8 ×1019 C, the Boltzmann constant k is 1.380 648 52 ×1023 J/K, 23 -1 the Avogadro constant NA is 6.022 140 857 ×10 mol , 12 the luminous efficacy of monochromatic radiation of frequency 540 ×10 hertz Kcd is 683 lm/W. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S.