Applied Spectroscopy Spectroscopic Nomenclature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Plant Lighting Basics and Applications
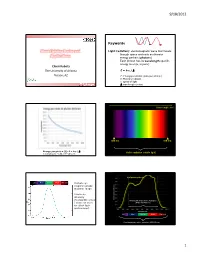
9/18/2012 Keywords Plant Lighting Basics and Light (radiation): electromagnetic wave that travels through space and exits as discrete Applications energy packets (photons) Each photon has its wavelength-specific energy level (E, in joule) Chieri Kubota The University of Arizona E = h·c / Tucson, AZ E: Energy per photon (joule per photon) h: Planck’s constant c: speed of light Greensys 2011, Greece : wavelength (meter) Wavelength (nm) 380 nm 780 nm Energy per photon [J]: E = h·c / Visible radiation (visible light) mole photons = 6.02 x 1023 photons Leaf photosynthesis UV Blue Green Red Far red Human eye response peaks at green range. Luminous intensity (footcandle or lux Photosynthetically Active Radiation ) does not work (PAR, 400-700 nm) for plant light environment. UV Blue Green Red Far red Plant biologically active radiation (300-800 nm) 1 9/18/2012 Chlorophyll a Phytochrome response Chlorophyll b UV Blue Green Red Far red Absorption spectra of isolated chlorophyll Absorptance 400 500 600 700 Wavelength (nm) Daily light integral (DLI or Daily Light Unit and Terminology PPF) Radiation Photons Visible light •Total amount of photosynthetically active “Base” unit Energy (J) Photons (mol) Luminous intensity (cd) radiation (400‐700 nm) received per sq Flux [total amount received Radiant flux Photon flux Luminous flux meter per day or emitted per time] (J s-1) or (W) (mol s-1) (lm) •Unit: mole per sq meter per day (mol m‐2 d‐1) Flux density [total amount Radiant flux density Photon flux (density) Illuminance, • Under optimal conditions, plant growth is received per area per time] (W m-2) (mol m-2 s-1) Luminous flux density highly correlated with DLI. -

Mixing of Quantum States: a New Route to Creating Optical Activity Anvar S
www.nature.com/scientificreports OPEN Mixing of quantum states: A new route to creating optical activity Anvar S. Baimuratov1, Nikita V. Tepliakov1, Yurii K. Gun’ko1,2, Alexander V. Baranov1, Anatoly V. Fedorov1 & Ivan D. Rukhlenko1,3 Received: 7 June 2016 The ability to induce optical activity in nanoparticles and dynamically control its strength is of great Accepted: 18 August 2016 practical importance due to potential applications in various areas, including biochemistry, toxicology, Published: xx xx xxxx and pharmaceutical science. Here we propose a new method of creating optical activity in originally achiral quantum nanostructures based on the mixing of their energy states of different parities. The mixing can be achieved by selective excitation of specific states orvia perturbing all the states in a controllable fashion. We analyze the general features of the so produced optical activity and elucidate the conditions required to realize the total dissymmetry of optical response. The proposed approach is applicable to a broad variety of real systems that can be used to advance chiroptical devices and methods. Chirality is known to occur at many levels of life organization and plays a key role in many chemical and biolog- ical processes in nature1. The fact that most of organic molecules are chiral starts more and more affecting the progress of contemporary medicine and pharmaceutical industry2. As a consequence, a great deal of research efforts has been recently focused on the optical activity of inherently chiral organic molecules3, 4. It can be strong in the ultraviolet range, in which case it is difficult to study and use in practice. -

CNT-Based Solar Thermal Coatings: Absorptance Vs. Emittance
coatings Article CNT-Based Solar Thermal Coatings: Absorptance vs. Emittance Yelena Vinetsky, Jyothi Jambu, Daniel Mandler * and Shlomo Magdassi * Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 9190401, Israel; [email protected] (Y.V.); [email protected] (J.J.) * Correspondence: [email protected] (D.M.); [email protected] (S.M.) Received: 15 October 2020; Accepted: 13 November 2020; Published: 17 November 2020 Abstract: A novel approach for fabricating selective absorbing coatings based on carbon nanotubes (CNTs) for mid-temperature solar–thermal application is presented. The developed formulations are dispersions of CNTs in water or solvents. Being coated on stainless steel (SS) by spraying, these formulations provide good characteristics of solar absorptance. The effect of CNT concentration and the type of the binder and its ratios to the CNT were investigated. Coatings based on water dispersions give higher adsorption, but solvent-based coatings enable achieving lower emittance. Interestingly, the binder was found to be responsible for the high emittance, yet, it is essential for obtaining good adhesion to the SS substrate. The best performance of the coatings requires adjusting the concentration of the CNTs and their ratio to the binder to obtain the highest absorptance with excellent adhesion; high absorptance is obtained at high CNT concentration, while good adhesion requires a minimum ratio between the binder/CNT; however, increasing the binder concentration increases the emissivity. The best coatings have an absorptance of ca. 90% with an emittance of ca. 0.3 and excellent adhesion to stainless steel. Keywords: carbon nanotubes (CNTs); binder; dispersion; solar thermal coating; absorptance; emittance; adhesion; selectivity 1. -

Polarization and Dichroism
Polarization and dichroism Amélie Juhin Sorbonne Université-CNRS (Paris) [email protected] 1 « Dichroism » (« two colors ») describes the dependence of the absorption measured with two orthogonal polarization states of the incoming light: Circular left Circular right k k ε ε Linear horizontal Linear vertical k k ε ε 2 By extension, « dichroism » also includes similar dependence phenomena, such as: • Low symmetry crystals show a trichroic dependence with linear light • Magneto-chiral dichroism (MχD) is measured with unpolarized light • Magnetic Linear Dichroism (MLD) is measured by changing the direction of magnetic ield and keeping the linear polarization ixed … Dichroism describes an angular and /or polarization behaviour of the absorption 3 Linear dichroism (LD) : difference measured with linearly polarized light Circular dichroism (CD) : difference measured with left / right circularly polarized light. Natural dichroism (ND) : time-reversal symmetry is conserved Non-Reciprocal (NR): time-reversal symmetry is not conserved Magnetic dichroism (MD) : measured in (ferro, ferri or antiferro) magnetic materials Dichroism Time reversal Parity symmetry symmetry Natural Linear (NLD) + + Magne6c Linear (MLD) + + Non Reciprocal Linear (NRLD) - - Natural Circular (NCD) + - Magne6c Circular (MCD) - + Magneto-op6cal (MχD) - - 4 The measurement of dichroism is often challenging… … but provides access to properties that cannot be measured in another way isotropic spectra polarized spectra 9 Al K edge Al K edge [6] beryl Al 6 6 corundum -Al O α -

Gem-Quality Tourmaline from LCT Pegmatite in Adamello Massif, Central Southern Alps, Italy: an Investigation of Its Mineralogy, Crystallography and 3D Inclusions
minerals Article Gem-Quality Tourmaline from LCT Pegmatite in Adamello Massif, Central Southern Alps, Italy: An Investigation of Its Mineralogy, Crystallography and 3D Inclusions Valeria Diella 1,* , Federico Pezzotta 2, Rosangela Bocchio 3, Nicoletta Marinoni 1,3, Fernando Cámara 3 , Antonio Langone 4 , Ilaria Adamo 5 and Gabriele Lanzafame 6 1 National Research Council, Institute for Dynamics of Environmental Processes (IDPA), Section of Milan, 20133 Milan, Italy; [email protected] 2 Natural History Museum, 20121 Milan, Italy; [email protected] 3 Department of Earth Sciences “Ardito Desio”, University of Milan, 20133 Milan, Italy; [email protected] (R.B.); [email protected] (F.C.) 4 National Research Council, Institute of Geosciences and Earth Resources (IGG), Section of Pavia, 27100 Pavia, Italy; [email protected] 5 Italian Gemmological Institute (IGI), 20123 Milan, Italy; [email protected] 6 Elettra-Sincrotrone Trieste S.C.p.A., Basovizza, 34149 Trieste, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-02-50315621 Received: 12 November 2018; Accepted: 7 December 2018; Published: 13 December 2018 Abstract: In the early 2000s, an exceptional discovery of gem-quality multi-coloured tourmalines, hosted in Litium-Cesium-Tantalum (LCT) pegmatites, was made in the Adamello Massif, Italy. Gem-quality tourmalines had never been found before in the Alps, and this new pegmatitic deposit is of particular interest and worthy of a detailed characterization. We studied a suite of faceted samples by classical gemmological methods, and fragments were studied with Synchrotron X-ray computed micro-tomography, which evidenced the occurrence of inclusions, cracks and voids. -

Direct Insolation Models
SERI/TR-33S-344 UC CATEGORY: UC-S9,61,62,63 DIRECT INSOLATION MODELS RICHARD BIRD ROLAND L. HULSTROM JANUARY 1980 PREPARED UNDER TASK No. 3623.01 Solar Energy Research Institute 1536 Cole Boulevard Golden. Colorado 80401 A Division of Midwest Research Institute Prepared for the U.S_ Department of Energy Contract No_ EG-77-C-01-4042 , '. Printed in the United States of America Available from: National Technical Information Service U.S. Departm ent of Commerce 5285 Port Royal Road Springfi e1dt VA 22161 Price: Microfiche $3.00 Printed Copy $ 5.25 NOTICE This report was prepared as an account of work sponsored by the United States Govern ment. Neither the United States nor the United States Department of Energy, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness or usefulness of any information, apparatus, product or process dis closed, or represents that its us e would not infringe privately owned rights. S=�I TR-344 FOREWORD This re port documents wo rk pe rforme d by the SERI Energy Resource Assessment Branch for the Division of Solar Energy Technology of the U.S. De pa rtment of Energy. The re po rt compares several simple direct insolation models with a rigorous solar transmission model and describes an improved , simple, direct insolation model. Roland L. Hulstrom , Branch Chief Energy Resource Assessment , \ Approved for: SOLAR ENERGY RE SEARCH INSTITUTE for Research iii S=�I TR-344 TABLE OF CONTENTS 1.0 Introduction •••••••••••••••••••••••••••••••••••••••••••••••••••••••• 1 2.0 Description of Models •••••••••••• . -

Investigation of Dichroism by Spectrophotometric Methods
Application Note Glass, Ceramics and Optics Investigation of Dichroism by Spectrophotometric Methods Authors Introduction N.S. Kozlova, E.V. Zabelina, Pleochroism (from ancient greek πλέον «more» + χρόμα «color») is an optical I.S. Didenko, A.P. Kozlova, phenomenon when a transparent crystal will have different colors if it is viewed from Zh.A. Goreeva, T different angles (1). Sometimes the color change is limited to shade changes such NUST “MISiS”, Russia as from pale pink to dark pink (2). Crystals are divided into optically isotropic (cubic crystal system), optically anisotropic uniaxial (hexagonal, trigonal, tetragonal crystal systems) and optically anisotropic biaxial (orthorhombic, monoclinic, triclinic crystal systems). The greatest change is limited to three colors. It may be observed in biaxial crystals and is called trichroic. A two color change may be observed in uniaxial crystals and called dichroic. Pleochroic is often the term used to cover both (2). Pleochroism is caused by optical anisotropy of the crystals Dichroism can be observed in non-polarized light but in (1-3). The absorption of light in the optically anisotropic polarized light it may be more pronounced if the plane of crystals depends on the frequency of the light wave and its polarization of incident light matches plane of polarization of polarization (direction of the electric vector in it) (3, 4). light that propagates in the crystal—ordinary or extraordinary Generally, any ray of light in the optical anisotropic crystal is wave. divided into two rays with perpendicular polarizations and The difference in absorbance of ray lights may be minor, but different velocities (v1, v2) which are inversely proportional to it may be significant and should be considered both when the refractive indices (n1, n2) (4). -

Lighting: the Principles
TECHNICAL GUIDE Cross Sector AHDB Fellowship CP 085 Dr Phillip Davis, Stockbridge Technology Centre Lighting: The principles Filmed over 3 weeks at Stockbridge Technology Centre SCAN WITH LAYAR APP 2 TECHNICAL GUIDE Lighting: The principles CONTENTS SECTION ONE LIGHT AND LIGHTING SECTION THREE LEDS IN HORTICULTURE 1.1 What is light? 6 3.1 Crop growth under red and blue LEDs 21 1.2 The natural light environment and its 3.2 The influences of other colours of impact on plant light responses 6 light on crop growth 22 1.3 The measurement of light 6 3.3 End of day, day extension and night interruption lighting 23 1.3.1 Photometric light measurement – lumens and lux 6 3.4 Interlighting 23 1.3.2 Radiometric light measurement – Watts per 3.5 Propagation 24 metre squared or Joules per metre squared 8 3.6 Improving crop quality 24 1.3.3 Photon counts – Moles 8 3.6.1 Improving crop morphology and reducing 1.3.4 Daily light integrals (DLI) 10 the use of plant growth regulators 24 1.4 Conversions between different 3.6.2 Improving pigmentation 25 measurement units 10 3.6.3 Improving flavour and aroma 26 1.5 Assessing light quality 10 3.7 Lighting strategies 26 1.6 Overview of light technologies 10 3.8 Other lighting techniques 27 1.6.1 Incandescent and halogen bulbs 10 3.9 Insect management under LEDs 27 1.6.2 Fluorescent tubes 10 3.10 Plant pathogens and their interactions 1.6.3 High intensity discharge lamps 11 with light 28 1.6.4 Plasma and sulphur lamps 12 3.11 Conclusions 28 1.6.5 LED technology 12 SECTION FOUR OTHER INFORMATION SECTION TWO PLANT LIGHT RESPONSES 4.1 References 30 2.1 Photosynthesis 15 2.2 Light stress – Photoinhibition 15 2.3 Sensing light quality 18 2.4 UVB light responses 18 2.5 Blue and UVA light responses 18 2.6 Green light responses 18 2.7 Red and far-red light responses 19 2.8 Hormones and light 19 3 TECHNICAL GUIDE Lighting: The principles GLOSSARY Cryptochrome A photoreceptor that is sensitive to blue and UVA light. -

Investigation of Magnetic Circular Dichroism Spectra of Semiconductor Quantum Rods and Quantum Dot-In-Rods
nanomaterials Article Investigation of Magnetic Circular Dichroism Spectra of Semiconductor Quantum Rods and Quantum Dot-in-Rods Farrukh Safin 1,* , Vladimir Maslov 1, Yulia Gromova 2, Ivan Korsakov 1, Ekaterina Kolesova 1, Aliaksei Dubavik 1, Sergei Cherevkov 1 and Yurii K. Gun’ko 2 1 School of Photonics, ITMO University, 197101 St. Petersburg, Russia; [email protected] (V.M.); [email protected] (I.K.); [email protected] (E.K.); [email protected] (A.D.); [email protected] (S.C.) 2 School of Chemistry, Trinity College, Dublin 2 Dublin, Ireland; [email protected] (Y.G.); [email protected] (Y.K.G.) * Correspondence: farruhsafi[email protected] Received: 20 April 2020; Accepted: 12 May 2020; Published: 30 May 2020 Abstract: Anisotropic quantum nanostructures have attracted a lot of attention due to their unique properties and a range of potential applications. Magnetic circular dichroism (MCD) spectra of semiconductor CdSe/ZnS Quantum Rods and CdSe/CdS Dot-in-Rods have been studied. Positions of four electronic transitions were determined by data fitting. MCD spectra were analyzed in the A and B terms, which characterize the splitting and mixing of states. Effective values of A and B terms were determined for each transition. A relatively high value of the B term is noted, which is most likely associated with the anisotropy of quantum rods. Keywords: magnetic circular dichroism; quantum nanocrystals; quantum rods; Dot-in-Rods 1. Introduction Anisotropic colloidal semiconductor nanocrystals of various shapes have attracted a lot of attention over recent years due to their unique properties and potential applications. -

Relation of Emittance to Other Optical Properties *
JOURNAL OF RESEARCH of the National Bureau of Standards-C. Engineering and Instrumentation Vol. 67C, No. 3, July- September 1963 Relation of Emittance to Other Optical Properties * J. C. Richmond (March 4, 19G3) An equation was derived relating t he normal spcctral e mittance of a n optically in homo geneous, partially transmitting coating appli ed over an opaque substrate t o t he t hi ckncs and optical properties of t he coating a nd the reflectance of thc substrate at thc coaLin g SLI bstrate interface. I. Introduction The 45 0 to 0 0 luminous daylight r efl ectance of a composite specimen com prising a parLiall:\' transparent, spectrally nonselective, ligh t-scattering coating applied to a completely opaque (nontransmitting) substrate, can be computed from the refl ecta.nce of the substrate, the thi ck ness of the coaLin g and the refl ectivity and coefficien t of scatter of the coating material. The equaLions derived [01' these condi tions have been found by experience [1 , 2)1 to be of considerable praeLical usefulness, even though sever al factors ar e known to exist in real m aterials that wer e no t consid ered in the derivation . For instance, porcelain enamels an d glossy paints have significant specular refl ectance at lhe coating-air interface, and no r ea'! coating is truly spectrally nonselective. The optical properties of a material vary with wavelength , but in the derivation of Lh e equations refened to above, they ar e consid ered to be independent of wavelength over the rather narrow wavelength band encompassin g visible ligh t. -

The Effects of Physical Parameters on the Absorption Coefficient of Natural Waters
AN ABSTRACT OF THE THESIS OF W. Scott Pegau for the degree of Doctor of Philosophy in Oceanography presented on February 23, 1996. Title: The Effects of Physical Parameters on the Absorption Coefficient of Natural Waters. Abstract approved: Redacted for privacy J. Ronald V. Zaneveld The absorption coefficient is one of the fundamental parameters used for studies in hydrologic optics. The significance of this parameter makes it important to understand how to properly measure it and how the absorption of components, such as water, depend on physical parameters such as temperature and salinity. A discussion is provided of a majority of the techniques available for measuring the absorption coefficient. Results from a comparison of the techniques indicates that natural variability in the optical properties is an important factor to consider when comparing or reporting measured values of the absorption coefficient. The agreement between methods was found to be best at longer wavelengths where the absorption by water is a significant portion of the total absorption coefficient. In order to test the accuracy of measurements of the absorption coefficient a practical TOP closure equation is developed. Application of the equation to field measurements is limited by our ability to measure the volume scattering function. The dependence of the absorption coefficient of water on temperature and salinity is investigated. It was found that the absorption coefficient in the near infrared portion of the spectrum is strongly dependent on temperature and salinity. In the visible region the only portion with a temperature dependence > 0.00 1 m1/°C is in the neighborhood of 610 nm. -

Copper-Bearing (Paraíba-Type) Tourmaline from Mozambique
COPPER-BEARING (PARAÍBA-TYPE) TOURMALINE FROM MOZAMBIQUE Brendan M. Laurs, J. C. (Hanco) Zwaan, Christopher M. Breeding, William B. (Skip) Simmons, Donna Beaton, Kenneth F. Rijsdijk, Riccardo Befi, and Alexander U. Falster Copper-bearing tourmaline from Mozambique was first recovered in 2001, but its Cu content was not recognized until 2003, and it was not widely sold with its Mozambique origin disclosed until 2005. It has been mined from alluvial deposits in an approximately 3 km2 area near Mavuco in the eastern portion of the Alto Ligonha pegmatite district. Most of the production has come from arti- sanal mining, with hand tools used to remove up to 5 m of overburden to reach the tourmaline- bearing layer. The stones exhibit a wide range of colors, typically pink to purple, violet to blue, and blue to green or yellowish green. Heat treatment of all but the green to yellowish green stones typi- cally produces Paraíba-like blue-to-green hues by reducing absorption at ~520 nm caused by the presence of Mn3+. The gemological properties are typical for Cu-bearing tourmaline (including material from Brazil and Nigeria); the most common inclusions consist of partially healed fractures and elongate hollow tubes. With the exception of some green to yellow-green stones, the tourma- lines examined have relatively low Cu contents and very low amounts of Fe and Ti. Mechanized mining is expected to increase production from this region in the near future. opper-bearing tourmaline, in bright blue- deposit of Cu-bearing tourmaline, and the “neon” to-green hues, is one of the most sought- blue and green shown by the finest stones closely after colored stones in the gem market.