Principles of Radiation Measurement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Plant Lighting Basics and Applications
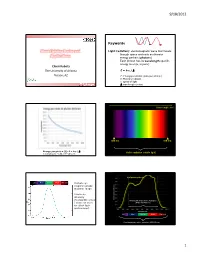
9/18/2012 Keywords Plant Lighting Basics and Light (radiation): electromagnetic wave that travels through space and exits as discrete Applications energy packets (photons) Each photon has its wavelength-specific energy level (E, in joule) Chieri Kubota The University of Arizona E = h·c / Tucson, AZ E: Energy per photon (joule per photon) h: Planck’s constant c: speed of light Greensys 2011, Greece : wavelength (meter) Wavelength (nm) 380 nm 780 nm Energy per photon [J]: E = h·c / Visible radiation (visible light) mole photons = 6.02 x 1023 photons Leaf photosynthesis UV Blue Green Red Far red Human eye response peaks at green range. Luminous intensity (footcandle or lux Photosynthetically Active Radiation ) does not work (PAR, 400-700 nm) for plant light environment. UV Blue Green Red Far red Plant biologically active radiation (300-800 nm) 1 9/18/2012 Chlorophyll a Phytochrome response Chlorophyll b UV Blue Green Red Far red Absorption spectra of isolated chlorophyll Absorptance 400 500 600 700 Wavelength (nm) Daily light integral (DLI or Daily Light Unit and Terminology PPF) Radiation Photons Visible light •Total amount of photosynthetically active “Base” unit Energy (J) Photons (mol) Luminous intensity (cd) radiation (400‐700 nm) received per sq Flux [total amount received Radiant flux Photon flux Luminous flux meter per day or emitted per time] (J s-1) or (W) (mol s-1) (lm) •Unit: mole per sq meter per day (mol m‐2 d‐1) Flux density [total amount Radiant flux density Photon flux (density) Illuminance, • Under optimal conditions, plant growth is received per area per time] (W m-2) (mol m-2 s-1) Luminous flux density highly correlated with DLI. -

Applied Spectroscopy Spectroscopic Nomenclature
Applied Spectroscopy Spectroscopic Nomenclature Absorbance, A Negative logarithm to the base 10 of the transmittance: A = –log10(T). (Not used: absorbancy, extinction, or optical density). (See Note 3). Absorptance, α Ratio of the radiant power absorbed by the sample to the incident radiant power; approximately equal to (1 – T). (See Notes 2 and 3). Absorption The absorption of electromagnetic radiation when light is transmitted through a medium; hence ‘‘absorption spectrum’’ or ‘‘absorption band’’. (Not used: ‘‘absorbance mode’’ or ‘‘absorbance band’’ or ‘‘absorbance spectrum’’ unless the ordinate axis of the spectrum is Absorbance.) (See Note 3). Absorption index, k See imaginary refractive index. Absorptivity, α Internal absorbance divided by the product of sample path length, ℓ , and mass concentration, ρ , of the absorbing material. A / α = i ρℓ SI unit: m2 kg–1. Common unit: cm2 g–1; L g–1 cm–1. (Not used: absorbancy index, extinction coefficient, or specific extinction.) Attenuated total reflection, ATR A sampling technique in which the evanescent wave of a beam that has been internally reflected from the internal surface of a material of high refractive index at an angle greater than the critical angle is absorbed by a sample that is held very close to the surface. (See Note 3.) Attenuation The loss of electromagnetic radiation caused by both absorption and scattering. Beer–Lambert law Absorptivity of a substance is constant with respect to changes in path length and concentration of the absorber. Often called Beer’s law when only changes in concentration are of interest. Brewster’s angle, θB The angle of incidence at which the reflection of p-polarized radiation is zero. -

Black Body Radiation and Radiometric Parameters
Black Body Radiation and Radiometric Parameters: All materials absorb and emit radiation to some extent. A blackbody is an idealization of how materials emit and absorb radiation. It can be used as a reference for real source properties. An ideal blackbody absorbs all incident radiation and does not reflect. This is true at all wavelengths and angles of incidence. Thermodynamic principals dictates that the BB must also radiate at all ’s and angles. The basic properties of a BB can be summarized as: 1. Perfect absorber/emitter at all ’s and angles of emission/incidence. Cavity BB 2. The total radiant energy emitted is only a function of the BB temperature. 3. Emits the maximum possible radiant energy from a body at a given temperature. 4. The BB radiation field does not depend on the shape of the cavity. The radiation field must be homogeneous and isotropic. T If the radiation going from a BB of one shape to another (both at the same T) were different it would cause a cooling or heating of one or the other cavity. This would violate the 1st Law of Thermodynamics. T T A B Radiometric Parameters: 1. Solid Angle dA d r 2 where dA is the surface area of a segment of a sphere surrounding a point. r d A r is the distance from the point on the source to the sphere. The solid angle looks like a cone with a spherical cap. z r d r r sind y r sin x An element of area of a sphere 2 dA rsin d d Therefore dd sin d The full solid angle surrounding a point source is: 2 dd sind 00 2cos 0 4 Or integrating to other angles < : 21cos The unit of solid angle is steradian. -

CNT-Based Solar Thermal Coatings: Absorptance Vs. Emittance
coatings Article CNT-Based Solar Thermal Coatings: Absorptance vs. Emittance Yelena Vinetsky, Jyothi Jambu, Daniel Mandler * and Shlomo Magdassi * Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 9190401, Israel; [email protected] (Y.V.); [email protected] (J.J.) * Correspondence: [email protected] (D.M.); [email protected] (S.M.) Received: 15 October 2020; Accepted: 13 November 2020; Published: 17 November 2020 Abstract: A novel approach for fabricating selective absorbing coatings based on carbon nanotubes (CNTs) for mid-temperature solar–thermal application is presented. The developed formulations are dispersions of CNTs in water or solvents. Being coated on stainless steel (SS) by spraying, these formulations provide good characteristics of solar absorptance. The effect of CNT concentration and the type of the binder and its ratios to the CNT were investigated. Coatings based on water dispersions give higher adsorption, but solvent-based coatings enable achieving lower emittance. Interestingly, the binder was found to be responsible for the high emittance, yet, it is essential for obtaining good adhesion to the SS substrate. The best performance of the coatings requires adjusting the concentration of the CNTs and their ratio to the binder to obtain the highest absorptance with excellent adhesion; high absorptance is obtained at high CNT concentration, while good adhesion requires a minimum ratio between the binder/CNT; however, increasing the binder concentration increases the emissivity. The best coatings have an absorptance of ca. 90% with an emittance of ca. 0.3 and excellent adhesion to stainless steel. Keywords: carbon nanotubes (CNTs); binder; dispersion; solar thermal coating; absorptance; emittance; adhesion; selectivity 1. -

Direct Insolation Models
SERI/TR-33S-344 UC CATEGORY: UC-S9,61,62,63 DIRECT INSOLATION MODELS RICHARD BIRD ROLAND L. HULSTROM JANUARY 1980 PREPARED UNDER TASK No. 3623.01 Solar Energy Research Institute 1536 Cole Boulevard Golden. Colorado 80401 A Division of Midwest Research Institute Prepared for the U.S_ Department of Energy Contract No_ EG-77-C-01-4042 , '. Printed in the United States of America Available from: National Technical Information Service U.S. Departm ent of Commerce 5285 Port Royal Road Springfi e1dt VA 22161 Price: Microfiche $3.00 Printed Copy $ 5.25 NOTICE This report was prepared as an account of work sponsored by the United States Govern ment. Neither the United States nor the United States Department of Energy, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness or usefulness of any information, apparatus, product or process dis closed, or represents that its us e would not infringe privately owned rights. S=�I TR-344 FOREWORD This re port documents wo rk pe rforme d by the SERI Energy Resource Assessment Branch for the Division of Solar Energy Technology of the U.S. De pa rtment of Energy. The re po rt compares several simple direct insolation models with a rigorous solar transmission model and describes an improved , simple, direct insolation model. Roland L. Hulstrom , Branch Chief Energy Resource Assessment , \ Approved for: SOLAR ENERGY RE SEARCH INSTITUTE for Research iii S=�I TR-344 TABLE OF CONTENTS 1.0 Introduction •••••••••••••••••••••••••••••••••••••••••••••••••••••••• 1 2.0 Description of Models •••••••••••• . -

Lighting: the Principles
TECHNICAL GUIDE Cross Sector AHDB Fellowship CP 085 Dr Phillip Davis, Stockbridge Technology Centre Lighting: The principles Filmed over 3 weeks at Stockbridge Technology Centre SCAN WITH LAYAR APP 2 TECHNICAL GUIDE Lighting: The principles CONTENTS SECTION ONE LIGHT AND LIGHTING SECTION THREE LEDS IN HORTICULTURE 1.1 What is light? 6 3.1 Crop growth under red and blue LEDs 21 1.2 The natural light environment and its 3.2 The influences of other colours of impact on plant light responses 6 light on crop growth 22 1.3 The measurement of light 6 3.3 End of day, day extension and night interruption lighting 23 1.3.1 Photometric light measurement – lumens and lux 6 3.4 Interlighting 23 1.3.2 Radiometric light measurement – Watts per 3.5 Propagation 24 metre squared or Joules per metre squared 8 3.6 Improving crop quality 24 1.3.3 Photon counts – Moles 8 3.6.1 Improving crop morphology and reducing 1.3.4 Daily light integrals (DLI) 10 the use of plant growth regulators 24 1.4 Conversions between different 3.6.2 Improving pigmentation 25 measurement units 10 3.6.3 Improving flavour and aroma 26 1.5 Assessing light quality 10 3.7 Lighting strategies 26 1.6 Overview of light technologies 10 3.8 Other lighting techniques 27 1.6.1 Incandescent and halogen bulbs 10 3.9 Insect management under LEDs 27 1.6.2 Fluorescent tubes 10 3.10 Plant pathogens and their interactions 1.6.3 High intensity discharge lamps 11 with light 28 1.6.4 Plasma and sulphur lamps 12 3.11 Conclusions 28 1.6.5 LED technology 12 SECTION FOUR OTHER INFORMATION SECTION TWO PLANT LIGHT RESPONSES 4.1 References 30 2.1 Photosynthesis 15 2.2 Light stress – Photoinhibition 15 2.3 Sensing light quality 18 2.4 UVB light responses 18 2.5 Blue and UVA light responses 18 2.6 Green light responses 18 2.7 Red and far-red light responses 19 2.8 Hormones and light 19 3 TECHNICAL GUIDE Lighting: The principles GLOSSARY Cryptochrome A photoreceptor that is sensitive to blue and UVA light. -

Relation of Emittance to Other Optical Properties *
JOURNAL OF RESEARCH of the National Bureau of Standards-C. Engineering and Instrumentation Vol. 67C, No. 3, July- September 1963 Relation of Emittance to Other Optical Properties * J. C. Richmond (March 4, 19G3) An equation was derived relating t he normal spcctral e mittance of a n optically in homo geneous, partially transmitting coating appli ed over an opaque substrate t o t he t hi ckncs and optical properties of t he coating a nd the reflectance of thc substrate at thc coaLin g SLI bstrate interface. I. Introduction The 45 0 to 0 0 luminous daylight r efl ectance of a composite specimen com prising a parLiall:\' transparent, spectrally nonselective, ligh t-scattering coating applied to a completely opaque (nontransmitting) substrate, can be computed from the refl ecta.nce of the substrate, the thi ck ness of the coaLin g and the refl ectivity and coefficien t of scatter of the coating material. The equaLions derived [01' these condi tions have been found by experience [1 , 2)1 to be of considerable praeLical usefulness, even though sever al factors ar e known to exist in real m aterials that wer e no t consid ered in the derivation . For instance, porcelain enamels an d glossy paints have significant specular refl ectance at lhe coating-air interface, and no r ea'! coating is truly spectrally nonselective. The optical properties of a material vary with wavelength , but in the derivation of Lh e equations refened to above, they ar e consid ered to be independent of wavelength over the rather narrow wavelength band encompassin g visible ligh t. -

Application Note (A2) LED Measurements
Application Note (A2) LED Measurements Revision: F JUNE 1997 OPTRONIC LABORATORIES, INC. 4632 36TH STREET ORLANDO, FLORIDA 32811 USA Tel: (407) 422-3171 Fax: (407) 648-5412 E-mail: www.olinet.com LED MEASUREMENTS Table of Contents 1.0 INTRODUCTION ................................................................................................................. 1 2.0 PHOTOMETRIC MEASUREMENTS.................................................................................... 1 2.1 Total Luminous Flux (lumens per 2B steradians)...................................................... 1 2.2 Luminous Intensity ( millicandelas) ........................................................................... 3 3.0 RADIOMETRIC MEASUREMENTS..................................................................................... 6 3.1 Total Radiant Flux or Power (watts per 2B steradians)............................................. 6 3.2 Radiant Intensity (watts per steradian) ..................................................................... 8 4.0 SPECTRORADIOMETRIC MEASUREMENTS.................................................................. 11 4.1 Spectral Radiant Flux or Power (watts per nm)....................................................... 11 4.2 Spectral Radiant Intensity (watts per steradian nm) ............................................... 13 4.3 Peak Wavelength and 50% Power Points............................................................... 15 LED MEASUREMENTS 1.0 INTRODUCTION The instrumentation selected to measure the output of an LED -

Radiometry of Light Emitting Diodes Table of Contents
TECHNICAL GUIDE THE RADIOMETRY OF LIGHT EMITTING DIODES TABLE OF CONTENTS 1.0 Introduction . .1 2.0 What is an LED? . .1 2.1 Device Physics and Package Design . .1 2.2 Electrical Properties . .3 2.2.1 Operation at Constant Current . .3 2.2.2 Modulated or Multiplexed Operation . .3 2.2.3 Single-Shot Operation . .3 3.0 Optical Characteristics of LEDs . .3 3.1 Spectral Properties of Light Emitting Diodes . .3 3.2 Comparison of Photometers and Spectroradiometers . .5 3.3 Color and Dominant Wavelength . .6 3.4 Influence of Temperature on Radiation . .6 4.0 Radiometric and Photopic Measurements . .7 4.1 Luminous and Radiant Intensity . .7 4.2 CIE 127 . .9 4.3 Spatial Distribution Characteristics . .10 4.4 Luminous Flux and Radiant Flux . .11 5.0 Terminology . .12 5.1 Radiometric Quantities . .12 5.2 Photometric Quantities . .12 6.0 References . .13 1.0 INTRODUCTION Almost everyone is familiar with light-emitting diodes (LEDs) from their use as indicator lights and numeric displays on consumer electronic devices. The low output and lack of color options of LEDs limited the technology to these uses for some time. New LED materials and improved production processes have produced bright LEDs in colors throughout the visible spectrum, including white light. With efficacies greater than incandescent (and approaching that of fluorescent lamps) along with their durability, small size, and light weight, LEDs are finding their way into many new applications within the lighting community. These new applications have placed increasingly stringent demands on the optical characterization of LEDs, which serves as the fundamental baseline for product quality and product design. -

Laser Terms Glossary
LASER TERMS GLOSSARY Texas State University – San Marcos 2/25/07 Risk Management & Safety LASER TERMS GLOSSARY Introduction - This section lists information pertinent to laser safety. The definitions in this glossary will not cover every term associated with lasers but does cover a majority of the terms. If a term should be encountered in your work with lasers and is not in this glossary, consult your supervisor or call the Texas State University Risk Management and Safety Office. Laser Terms ABSORPTION: means the transformation of radiant energy to a different form by interaction with matter. ACCESS CONTROL: Entry must be restricted to authorized laser personnel during the operation of laser equipment. ACCESSIBLE EMISSION LIMIT (AEL): - means the maximum accessible emission level permitted within a particular class. WAVELENGTH DURATION CLASS 1 CLASS 2 CLASS 3a CLASS 3b CLASS 4 (μm) (s) (W) (W) (W) (W) (W) Ultraviolet 0.18 to 0.302 3x104 ≤ 9.6x10-9 - Between 1 to 5 > Class 3a but > 0.5 0.302 to 0.4 3x104 ≤ 3.2x10-6 times Class 1 ≤ 0.5 Visible > Class 1 Less than 5 > Class 3a but 0.4 to 0.7 10 ≤ 0.4x10-3 > 0.5 but < 0.001 times Class 2 ≤ 0.5 Near IR ≤ 0.4x10-3 Between 1 to 5 > Class 3a but 0.7 to 1.05 ≥ 10 - > 0.5 to <1.9x10-3 times Class 1 ≤ 0.5 IR 1.05 to 1.15 ≤ 1.9x10-3 ≤ 1.9x10-3 Between 1 to 5 > Class 3a but 1.15 to 1.2 ≥ 10 - > 0.5 to 1.5x10-2 times Class 1 ≤ 0.5 1.2 to 1.4 ≤ 1.5x10-2 Far IR Between 1 to 5 > Class 3a but 1.4 to 100 ≥ 10 ≤ 9.6x10-3 - > 0.5 times Class 1 ≤ 0.5 AGENCY: means the Texas Department of State Health Services Radiation Control agency. -

The Effects of Physical Parameters on the Absorption Coefficient of Natural Waters
AN ABSTRACT OF THE THESIS OF W. Scott Pegau for the degree of Doctor of Philosophy in Oceanography presented on February 23, 1996. Title: The Effects of Physical Parameters on the Absorption Coefficient of Natural Waters. Abstract approved: Redacted for privacy J. Ronald V. Zaneveld The absorption coefficient is one of the fundamental parameters used for studies in hydrologic optics. The significance of this parameter makes it important to understand how to properly measure it and how the absorption of components, such as water, depend on physical parameters such as temperature and salinity. A discussion is provided of a majority of the techniques available for measuring the absorption coefficient. Results from a comparison of the techniques indicates that natural variability in the optical properties is an important factor to consider when comparing or reporting measured values of the absorption coefficient. The agreement between methods was found to be best at longer wavelengths where the absorption by water is a significant portion of the total absorption coefficient. In order to test the accuracy of measurements of the absorption coefficient a practical TOP closure equation is developed. Application of the equation to field measurements is limited by our ability to measure the volume scattering function. The dependence of the absorption coefficient of water on temperature and salinity is investigated. It was found that the absorption coefficient in the near infrared portion of the spectrum is strongly dependent on temperature and salinity. In the visible region the only portion with a temperature dependence > 0.00 1 m1/°C is in the neighborhood of 610 nm. -

Radiometry and Photometry
Radiometry and Photometry Wei-Chih Wang Department of Power Mechanical Engineering National TsingHua University W. Wang Materials Covered • Radiometry - Radiant Flux - Radiant Intensity - Irradiance - Radiance • Photometry - luminous Flux - luminous Intensity - Illuminance - luminance Conversion from radiometric and photometric W. Wang Radiometry Radiometry is the detection and measurement of light waves in the optical portion of the electromagnetic spectrum which is further divided into ultraviolet, visible, and infrared light. Example of a typical radiometer 3 W. Wang Photometry All light measurement is considered radiometry with photometry being a special subset of radiometry weighted for a typical human eye response. Example of a typical photometer 4 W. Wang Human Eyes Figure shows a schematic illustration of the human eye (Encyclopedia Britannica, 1994). The inside of the eyeball is clad by the retina, which is the light-sensitive part of the eye. The illustration also shows the fovea, a cone-rich central region of the retina which affords the high acuteness of central vision. Figure also shows the cell structure of the retina including the light-sensitive rod cells and cone cells. Also shown are the ganglion cells and nerve fibers that transmit the visual information to the brain. Rod cells are more abundant and more light sensitive than cone cells. Rods are 5 sensitive over the entire visible spectrum. W. Wang There are three types of cone cells, namely cone cells sensitive in the red, green, and blue spectral range. The approximate spectral sensitivity functions of the rods and three types or cones are shown in the figure above 6 W. Wang Eye sensitivity function The conversion between radiometric and photometric units is provided by the luminous efficiency function or eye sensitivity function, V(λ).