Radiometric Quantities and Units Used in Photobiology And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Plant Lighting Basics and Applications
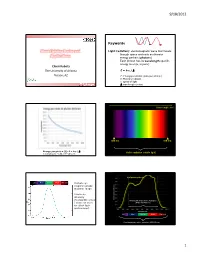
9/18/2012 Keywords Plant Lighting Basics and Light (radiation): electromagnetic wave that travels through space and exits as discrete Applications energy packets (photons) Each photon has its wavelength-specific energy level (E, in joule) Chieri Kubota The University of Arizona E = h·c / Tucson, AZ E: Energy per photon (joule per photon) h: Planck’s constant c: speed of light Greensys 2011, Greece : wavelength (meter) Wavelength (nm) 380 nm 780 nm Energy per photon [J]: E = h·c / Visible radiation (visible light) mole photons = 6.02 x 1023 photons Leaf photosynthesis UV Blue Green Red Far red Human eye response peaks at green range. Luminous intensity (footcandle or lux Photosynthetically Active Radiation ) does not work (PAR, 400-700 nm) for plant light environment. UV Blue Green Red Far red Plant biologically active radiation (300-800 nm) 1 9/18/2012 Chlorophyll a Phytochrome response Chlorophyll b UV Blue Green Red Far red Absorption spectra of isolated chlorophyll Absorptance 400 500 600 700 Wavelength (nm) Daily light integral (DLI or Daily Light Unit and Terminology PPF) Radiation Photons Visible light •Total amount of photosynthetically active “Base” unit Energy (J) Photons (mol) Luminous intensity (cd) radiation (400‐700 nm) received per sq Flux [total amount received Radiant flux Photon flux Luminous flux meter per day or emitted per time] (J s-1) or (W) (mol s-1) (lm) •Unit: mole per sq meter per day (mol m‐2 d‐1) Flux density [total amount Radiant flux density Photon flux (density) Illuminance, • Under optimal conditions, plant growth is received per area per time] (W m-2) (mol m-2 s-1) Luminous flux density highly correlated with DLI. -

Fundametals of Rendering - Radiometry / Photometry
Fundametals of Rendering - Radiometry / Photometry “Physically Based Rendering” by Pharr & Humphreys •Chapter 5: Color and Radiometry •Chapter 6: Camera Models - we won’t cover this in class 782 Realistic Rendering • Determination of Intensity • Mechanisms – Emittance (+) – Absorption (-) – Scattering (+) (single vs. multiple) • Cameras or retinas record quantity of light 782 Pertinent Questions • Nature of light and how it is: – Measured – Characterized / recorded • (local) reflection of light • (global) spatial distribution of light 782 Electromagnetic spectrum 782 Spectral Power Distributions e.g., Fluorescent Lamps 782 Tristimulus Theory of Color Metamers: SPDs that appear the same visually Color matching functions of standard human observer International Commision on Illumination, or CIE, of 1931 “These color matching functions are the amounts of three standard monochromatic primaries needed to match the monochromatic test primary at the wavelength shown on the horizontal scale.” from Wikipedia “CIE 1931 Color Space” 782 Optics Three views •Geometrical or ray – Traditional graphics – Reflection, refraction – Optical system design •Physical or wave – Dispersion, interference – Interaction of objects of size comparable to wavelength •Quantum or photon optics – Interaction of light with atoms and molecules 782 What Is Light ? • Light - particle model (Newton) – Light travels in straight lines – Light can travel through a vacuum (waves need a medium to travel in) – Quantum amount of energy • Light – wave model (Huygens): electromagnetic radiation: sinusiodal wave formed coupled electric (E) and magnetic (H) fields 782 Nature of Light • Wave-particle duality – Light has some wave properties: frequency, phase, orientation – Light has some quantum particle properties: quantum packets (photons). • Dimensions of light – Amplitude or Intensity – Frequency – Phase – Polarization 782 Nature of Light • Coherence - Refers to frequencies of waves • Laser light waves have uniform frequency • Natural light is incoherent- waves are multiple frequencies, and random in phase. -

Far Infrared Radiation Exposure
INTERNATIONAL COMMISSION ON NON‐IONIZING RADIATION PROTECTION ICNIRP STATEMENT ON FAR INFRARED RADIATION EXPOSURE PUBLISHED IN: HEALTH PHYSICS 91(6):630‐645; 2006 ICNIRP PUBLICATION – 2006 ICNIRP Statement ICNIRP STATEMENT ON FAR INFRARED RADIATION EXPOSURE The International Commission on Non-Ionizing Radiation Protection* INTRODUCTION the health hazards associated with these hot environ- ments. Heat strain and discomfort (thermal pain) nor- THE INTERNATIONAL Commission on Non Ionizing Radia- mally limit skin exposure to infrared radiation levels tion Protection (ICNIRP) currently provides guidelines below the threshold for skin-thermal injury, and this is to limit human exposure to intense, broadband infrared particularly true for sources that emit largely IR-C. radiation (ICNIRP 1997). The guidelines that pertained Furthermore, limits for lengthy infrared exposures would to infrared radiation (IR) were developed initially with an have to consider ambient temperatures. For example, an aim to provide guidance for protecting against hazards infrared irradiance of 1 kW mϪ2 (100 mW cmϪ2)atan from high-intensity artificial sources and to protect work- ambient temperature of 5°C can be comfortably warm- ers in hot industries. Detailed guidance for exposure to ing, but at an ambient temperature of 30°C this irradiance longer far-infrared wavelengths (referred to as IR-C would be painful and produce severe heat strain. There- radiation) was not provided because the energy at longer fore, ICNIRP provided guidelines to limit skin exposure wavelengths from most lamps and industrial infrared to pulsed sources and very brief exposures where thermal sources of concern actually contribute only a small injury could take place faster than the pain response time fraction of the total radiant heat energy and did not and where environmental temperature and the irradiated require measurement. -

About SI Units
Units SI units: International system of units (French: “SI” means “Système Internationale”) The seven SI base units are: Mass: kg Defined by the prototype “standard kilogram” located in Paris. The standard kilogram is made of Pt metal. Length: m Originally defined by the prototype “standard meter” located in Paris. Then defined as 1,650,763.73 wavelengths of the orange-red radiation of Krypton86 under certain specified conditions. (Official definition: The distance traveled by light in vacuum during a time interval of 1 / 299 792 458 of a second) Time: s The second is defined as the duration of a certain number of oscillations of radiation coming from Cs atoms. (Official definition: The second is the duration of 9,192,631,770 periods of the radiation of the hyperfine- level transition of the ground state of the Cs133 atom) Current: A Defined as the current that causes a certain force between two parallel wires. (Official definition: The ampere is that constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross-section, and placed 1 meter apart in vacuum, would produce between these conductors a force equal to 2 × 10-7 Newton per meter of length. Temperature: K One percent of the temperature difference between boiling point and freezing point of water. (Official definition: The Kelvin, unit of thermodynamic temperature, is the fraction 1 / 273.16 of the thermodynamic temperature of the triple point of water. Amount of substance: mol The amount of a substance that contains Avogadro’s number NAvo = 6.0221 × 1023 of atoms or molecules. -

Applied Spectroscopy Spectroscopic Nomenclature
Applied Spectroscopy Spectroscopic Nomenclature Absorbance, A Negative logarithm to the base 10 of the transmittance: A = –log10(T). (Not used: absorbancy, extinction, or optical density). (See Note 3). Absorptance, α Ratio of the radiant power absorbed by the sample to the incident radiant power; approximately equal to (1 – T). (See Notes 2 and 3). Absorption The absorption of electromagnetic radiation when light is transmitted through a medium; hence ‘‘absorption spectrum’’ or ‘‘absorption band’’. (Not used: ‘‘absorbance mode’’ or ‘‘absorbance band’’ or ‘‘absorbance spectrum’’ unless the ordinate axis of the spectrum is Absorbance.) (See Note 3). Absorption index, k See imaginary refractive index. Absorptivity, α Internal absorbance divided by the product of sample path length, ℓ , and mass concentration, ρ , of the absorbing material. A / α = i ρℓ SI unit: m2 kg–1. Common unit: cm2 g–1; L g–1 cm–1. (Not used: absorbancy index, extinction coefficient, or specific extinction.) Attenuated total reflection, ATR A sampling technique in which the evanescent wave of a beam that has been internally reflected from the internal surface of a material of high refractive index at an angle greater than the critical angle is absorbed by a sample that is held very close to the surface. (See Note 3.) Attenuation The loss of electromagnetic radiation caused by both absorption and scattering. Beer–Lambert law Absorptivity of a substance is constant with respect to changes in path length and concentration of the absorber. Often called Beer’s law when only changes in concentration are of interest. Brewster’s angle, θB The angle of incidence at which the reflection of p-polarized radiation is zero. -

CNT-Based Solar Thermal Coatings: Absorptance Vs. Emittance
coatings Article CNT-Based Solar Thermal Coatings: Absorptance vs. Emittance Yelena Vinetsky, Jyothi Jambu, Daniel Mandler * and Shlomo Magdassi * Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 9190401, Israel; [email protected] (Y.V.); [email protected] (J.J.) * Correspondence: [email protected] (D.M.); [email protected] (S.M.) Received: 15 October 2020; Accepted: 13 November 2020; Published: 17 November 2020 Abstract: A novel approach for fabricating selective absorbing coatings based on carbon nanotubes (CNTs) for mid-temperature solar–thermal application is presented. The developed formulations are dispersions of CNTs in water or solvents. Being coated on stainless steel (SS) by spraying, these formulations provide good characteristics of solar absorptance. The effect of CNT concentration and the type of the binder and its ratios to the CNT were investigated. Coatings based on water dispersions give higher adsorption, but solvent-based coatings enable achieving lower emittance. Interestingly, the binder was found to be responsible for the high emittance, yet, it is essential for obtaining good adhesion to the SS substrate. The best performance of the coatings requires adjusting the concentration of the CNTs and their ratio to the binder to obtain the highest absorptance with excellent adhesion; high absorptance is obtained at high CNT concentration, while good adhesion requires a minimum ratio between the binder/CNT; however, increasing the binder concentration increases the emissivity. The best coatings have an absorptance of ca. 90% with an emittance of ca. 0.3 and excellent adhesion to stainless steel. Keywords: carbon nanotubes (CNTs); binder; dispersion; solar thermal coating; absorptance; emittance; adhesion; selectivity 1. -

Direct Insolation Models
SERI/TR-33S-344 UC CATEGORY: UC-S9,61,62,63 DIRECT INSOLATION MODELS RICHARD BIRD ROLAND L. HULSTROM JANUARY 1980 PREPARED UNDER TASK No. 3623.01 Solar Energy Research Institute 1536 Cole Boulevard Golden. Colorado 80401 A Division of Midwest Research Institute Prepared for the U.S_ Department of Energy Contract No_ EG-77-C-01-4042 , '. Printed in the United States of America Available from: National Technical Information Service U.S. Departm ent of Commerce 5285 Port Royal Road Springfi e1dt VA 22161 Price: Microfiche $3.00 Printed Copy $ 5.25 NOTICE This report was prepared as an account of work sponsored by the United States Govern ment. Neither the United States nor the United States Department of Energy, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness or usefulness of any information, apparatus, product or process dis closed, or represents that its us e would not infringe privately owned rights. S=�I TR-344 FOREWORD This re port documents wo rk pe rforme d by the SERI Energy Resource Assessment Branch for the Division of Solar Energy Technology of the U.S. De pa rtment of Energy. The re po rt compares several simple direct insolation models with a rigorous solar transmission model and describes an improved , simple, direct insolation model. Roland L. Hulstrom , Branch Chief Energy Resource Assessment , \ Approved for: SOLAR ENERGY RE SEARCH INSTITUTE for Research iii S=�I TR-344 TABLE OF CONTENTS 1.0 Introduction •••••••••••••••••••••••••••••••••••••••••••••••••••••••• 1 2.0 Description of Models •••••••••••• . -

Lighting: the Principles
TECHNICAL GUIDE Cross Sector AHDB Fellowship CP 085 Dr Phillip Davis, Stockbridge Technology Centre Lighting: The principles Filmed over 3 weeks at Stockbridge Technology Centre SCAN WITH LAYAR APP 2 TECHNICAL GUIDE Lighting: The principles CONTENTS SECTION ONE LIGHT AND LIGHTING SECTION THREE LEDS IN HORTICULTURE 1.1 What is light? 6 3.1 Crop growth under red and blue LEDs 21 1.2 The natural light environment and its 3.2 The influences of other colours of impact on plant light responses 6 light on crop growth 22 1.3 The measurement of light 6 3.3 End of day, day extension and night interruption lighting 23 1.3.1 Photometric light measurement – lumens and lux 6 3.4 Interlighting 23 1.3.2 Radiometric light measurement – Watts per 3.5 Propagation 24 metre squared or Joules per metre squared 8 3.6 Improving crop quality 24 1.3.3 Photon counts – Moles 8 3.6.1 Improving crop morphology and reducing 1.3.4 Daily light integrals (DLI) 10 the use of plant growth regulators 24 1.4 Conversions between different 3.6.2 Improving pigmentation 25 measurement units 10 3.6.3 Improving flavour and aroma 26 1.5 Assessing light quality 10 3.7 Lighting strategies 26 1.6 Overview of light technologies 10 3.8 Other lighting techniques 27 1.6.1 Incandescent and halogen bulbs 10 3.9 Insect management under LEDs 27 1.6.2 Fluorescent tubes 10 3.10 Plant pathogens and their interactions 1.6.3 High intensity discharge lamps 11 with light 28 1.6.4 Plasma and sulphur lamps 12 3.11 Conclusions 28 1.6.5 LED technology 12 SECTION FOUR OTHER INFORMATION SECTION TWO PLANT LIGHT RESPONSES 4.1 References 30 2.1 Photosynthesis 15 2.2 Light stress – Photoinhibition 15 2.3 Sensing light quality 18 2.4 UVB light responses 18 2.5 Blue and UVA light responses 18 2.6 Green light responses 18 2.7 Red and far-red light responses 19 2.8 Hormones and light 19 3 TECHNICAL GUIDE Lighting: The principles GLOSSARY Cryptochrome A photoreceptor that is sensitive to blue and UVA light. -

Glossary of Terms
GLOSSARY OF TERMS Terminology Used for Ultraviolet (UV) Curing Process Design and Measurement This glossary of terms has been assembled in order to provide users, formulators, suppliers and researchers with terms that are used in the design and measurement of UV curing systems. It was prompted by the scattered and sometimes incorrect terms used in industrial UV curing technologies. It is intended to provide common and technical meanings as used in and appropriate for UV process design, measurement, and specification. General scientific terms are included only where they relate to UV Measurements. The object is to be "user-friendly," with descriptions and comments on meaning and usage, and minimum use of mathematical and strict definitions, but technically correct. Occasionally, where two or more terms are used similarly, notes will indicate the preferred term. For historical and other reasons, terms applicable to UV Curing may vary slightly in their usage from other sciences. This glossary is intended to 'close the gap' in technical language, and is recommended for authors, suppliers and designers in UV Curing technologies. absorbance An index of the light or UV absorbed by a medium compared to the light transmitted through it. Numerically, it is the logarithm of the ratio of incident spectral irradiance to the transmitted spectral irradiance. It is unitless number. Absorbance implies monochromatic radiation, although it is sometimes used as an average applied over a specified wavelength range. absorptivity (absorption coefficient) Absorbance per unit thickness of a medium. actinometer A chemical system or physical device that determines the number of photons in a beam integrally or per unit time. -

Relation of Emittance to Other Optical Properties *
JOURNAL OF RESEARCH of the National Bureau of Standards-C. Engineering and Instrumentation Vol. 67C, No. 3, July- September 1963 Relation of Emittance to Other Optical Properties * J. C. Richmond (March 4, 19G3) An equation was derived relating t he normal spcctral e mittance of a n optically in homo geneous, partially transmitting coating appli ed over an opaque substrate t o t he t hi ckncs and optical properties of t he coating a nd the reflectance of thc substrate at thc coaLin g SLI bstrate interface. I. Introduction The 45 0 to 0 0 luminous daylight r efl ectance of a composite specimen com prising a parLiall:\' transparent, spectrally nonselective, ligh t-scattering coating applied to a completely opaque (nontransmitting) substrate, can be computed from the refl ecta.nce of the substrate, the thi ck ness of the coaLin g and the refl ectivity and coefficien t of scatter of the coating material. The equaLions derived [01' these condi tions have been found by experience [1 , 2)1 to be of considerable praeLical usefulness, even though sever al factors ar e known to exist in real m aterials that wer e no t consid ered in the derivation . For instance, porcelain enamels an d glossy paints have significant specular refl ectance at lhe coating-air interface, and no r ea'! coating is truly spectrally nonselective. The optical properties of a material vary with wavelength , but in the derivation of Lh e equations refened to above, they ar e consid ered to be independent of wavelength over the rather narrow wavelength band encompassin g visible ligh t. -

Application Note (A2) LED Measurements
Application Note (A2) LED Measurements Revision: F JUNE 1997 OPTRONIC LABORATORIES, INC. 4632 36TH STREET ORLANDO, FLORIDA 32811 USA Tel: (407) 422-3171 Fax: (407) 648-5412 E-mail: www.olinet.com LED MEASUREMENTS Table of Contents 1.0 INTRODUCTION ................................................................................................................. 1 2.0 PHOTOMETRIC MEASUREMENTS.................................................................................... 1 2.1 Total Luminous Flux (lumens per 2B steradians)...................................................... 1 2.2 Luminous Intensity ( millicandelas) ........................................................................... 3 3.0 RADIOMETRIC MEASUREMENTS..................................................................................... 6 3.1 Total Radiant Flux or Power (watts per 2B steradians)............................................. 6 3.2 Radiant Intensity (watts per steradian) ..................................................................... 8 4.0 SPECTRORADIOMETRIC MEASUREMENTS.................................................................. 11 4.1 Spectral Radiant Flux or Power (watts per nm)....................................................... 11 4.2 Spectral Radiant Intensity (watts per steradian nm) ............................................... 13 4.3 Peak Wavelength and 50% Power Points............................................................... 15 LED MEASUREMENTS 1.0 INTRODUCTION The instrumentation selected to measure the output of an LED -

Princeton University Laser Safety Training Guide
Laser Safety Training Guide Environmental Health and Safety http://www.princeton.edu/ehs September 2007 LASER SAFETY TRAINING GUIDE TABLE OF CONTENTS SECTION 1: LASER FUNDAMENTALS 3 LASER Theory And Operation 4 Components Of A Laser 5 Types Of Lasers 6 SECTION 2: LASER HAZARDS 8 BEAM-RELATED HAZARDS 8 Types of Beam Exposure 9 Eye 9 Skin 12 NON-BEAM HAZARDS 12 Electrical Hazards 13 Laser Generated Air Contaminants -- The “Plume” 14 Collateral and Plasma Radiation 14 Fire Hazards 15 Compressed Gases 15 Laser Dyes 15 SECTION 3: LASER HAZARD CLASSIFICATION 17 SECTION 4: LASER CONTROL MEASURES 19 Maximum Permissible Exposure (MPE) 19 Accessible Exposrue Limit 19 Optical Density (OD) 19 Nominal Hazard Zone (NHZ) 20 Control Measures by Laser Classification 21 Protective Equipment 27 Protective Eyewear 27 Laser Eye Protection Selection Process 27 Other Protective Equipment 28 Special Controls for UltraViolet and Infrared Lasers 29 SECTION 5: LASER SAFETY AT PRINCETON UNIVERSITY 30 SECTION 6: GLOSSARY 33 APPENDIX A: SELECTED ANSI STANDARD TABLES 36 APPENDIX B: LASER SAFETY TIPS 43 APPENDIX C: LASER SAFETY CHECKLIST 44 2 Section 1: LASER FUNDAMENTALS Introduction The word laser is an acronym for Light Amplification by Stimulated Emission of Radiation. Lasers are used as research aides in many departments at Princeton University. In this document, the word laser will be limited to electromagnetic radiation-emitting devices using light amplification by stimulated emission of radiation at wavelengths from 180 nanometers to 1 millimeter. The electromagnetic spectrum includes energy ranging from gamma rays to electricity. Figure 1 illustrates the total electromagnetic spectrum and wavelengths of the various regions.