Radiometry of Light Emitting Diodes Table of Contents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -

LED Lighting Standards in ENERGY STAR Programs
LED Lig hting Standards in ENERGY STAR® Programs Jianzhong Jiao, Ph.D. OSRAM Opto Semiconductors Inc. Outline Introduction LED lighting standards referred to or referenced in ENERGY STAR® Specs –ANSI – IESNA – UL –NEMA Implementations of LED lighting standards in ENERGY STAR® Programs – Testing for qualifications – Considerations of new standards EPA Energy Star Products Partner Meeting | Nov. 9, 2011 | 2 Introduction LED Lighting Standardization Bodies in USA Professional associations – SAE (Society of Automotive Engineers) – IESNA (Illumination Engineering Society North America) – IEEE-SA (Institute of Electrical and Electronics Engineers Standards Association) Standard organizations – UL (Underwriter Laboratories) – ANSI (American National Standard Institute) Trade associations – NEMA (National Electrical Manufacturers Association) – JEDEC (Joint Electron Device Engineering Council) – SEMI (Semiconductor Equipment and Materials International) EPA Energy Star Products Partner Meeting | Nov. 9, 2011 | 3 Introduction U.S. Standard Organizations ISO General Lighting ANSI CIE IEC GTB SAE IEEE IESNA NEMA UL ITE JEDEC USA SEMI EPA Energy Star Products Partner Meeting | Nov. 9, 2011 | 4 Introduction Government Regulations or Specifications for LED Lighting Federal government: DOT, EPA, DOE, … – Rule making (establish rules by federal agencies) – Enforcement – “Endorsement”, approval, labeling Federal government: DOC – National Institute of Standards and Technology (NIST): US National Metrology Institute – Promote US innovation and -

Fundametals of Rendering - Radiometry / Photometry
Fundametals of Rendering - Radiometry / Photometry “Physically Based Rendering” by Pharr & Humphreys •Chapter 5: Color and Radiometry •Chapter 6: Camera Models - we won’t cover this in class 782 Realistic Rendering • Determination of Intensity • Mechanisms – Emittance (+) – Absorption (-) – Scattering (+) (single vs. multiple) • Cameras or retinas record quantity of light 782 Pertinent Questions • Nature of light and how it is: – Measured – Characterized / recorded • (local) reflection of light • (global) spatial distribution of light 782 Electromagnetic spectrum 782 Spectral Power Distributions e.g., Fluorescent Lamps 782 Tristimulus Theory of Color Metamers: SPDs that appear the same visually Color matching functions of standard human observer International Commision on Illumination, or CIE, of 1931 “These color matching functions are the amounts of three standard monochromatic primaries needed to match the monochromatic test primary at the wavelength shown on the horizontal scale.” from Wikipedia “CIE 1931 Color Space” 782 Optics Three views •Geometrical or ray – Traditional graphics – Reflection, refraction – Optical system design •Physical or wave – Dispersion, interference – Interaction of objects of size comparable to wavelength •Quantum or photon optics – Interaction of light with atoms and molecules 782 What Is Light ? • Light - particle model (Newton) – Light travels in straight lines – Light can travel through a vacuum (waves need a medium to travel in) – Quantum amount of energy • Light – wave model (Huygens): electromagnetic radiation: sinusiodal wave formed coupled electric (E) and magnetic (H) fields 782 Nature of Light • Wave-particle duality – Light has some wave properties: frequency, phase, orientation – Light has some quantum particle properties: quantum packets (photons). • Dimensions of light – Amplitude or Intensity – Frequency – Phase – Polarization 782 Nature of Light • Coherence - Refers to frequencies of waves • Laser light waves have uniform frequency • Natural light is incoherent- waves are multiple frequencies, and random in phase. -

Light and Illumination
ChapterChapter 3333 -- LightLight andand IlluminationIllumination AAA PowerPointPowerPointPowerPoint PresentationPresentationPresentation bybyby PaulPaulPaul E.E.E. Tippens,Tippens,Tippens, ProfessorProfessorProfessor ofofof PhysicsPhysicsPhysics SouthernSouthernSouthern PolytechnicPolytechnicPolytechnic StateStateState UniversityUniversityUniversity © 2007 Objectives:Objectives: AfterAfter completingcompleting thisthis module,module, youyou shouldshould bebe ableable to:to: •• DefineDefine lightlight,, discussdiscuss itsits properties,properties, andand givegive thethe rangerange ofof wavelengthswavelengths forfor visiblevisible spectrum.spectrum. •• ApplyApply thethe relationshiprelationship betweenbetween frequenciesfrequencies andand wavelengthswavelengths forfor opticaloptical waves.waves. •• DefineDefine andand applyapply thethe conceptsconcepts ofof luminousluminous fluxflux,, luminousluminous intensityintensity,, andand illuminationillumination.. •• SolveSolve problemsproblems similarsimilar toto thosethose presentedpresented inin thisthis module.module. AA BeginningBeginning DefinitionDefinition AllAll objectsobjects areare emittingemitting andand absorbingabsorbing EMEM radiaradia-- tiontion.. ConsiderConsider aa pokerpoker placedplaced inin aa fire.fire. AsAs heatingheating occurs,occurs, thethe 1 emittedemitted EMEM waveswaves havehave 2 higherhigher energyenergy andand 3 eventuallyeventually becomebecome visible.visible. 4 FirstFirst redred .. .. .. thenthen white.white. LightLightLight maymaymay bebebe defineddefineddefined -

Chapter 4 Reflected Light Optics
l CHAPTER 4 REFLECTED LIGHT OPTICS 4.1 INTRODUCTION Light is a form ofelectromagnetic radiation. which may be emitted by matter that is in a suitably "energized" (excited) state (e.g.,the tungsten filament ofa microscope lamp emits light when "energized" by the passage of an electric current). One ofthe interesting consequences ofthe developments in physics in the early part of the twentieth century was the realization that light and other forms of electromagnetic radiation can be described both as waves and as a stream ofparticles (photons). These are not conflicting theories but rather complementary ways of describing light; in different circumstances. either one may be the more appropriate. For most aspects ofmicroscope optics. the "classical" approach ofdescribing light as waves is more applicable. However, particularly (as outlined in Chapter 5) when the relationship between the reflecting process and the structure and composition ofa solid is considered. it is useful to regard light as photons. The electromagnetic radiation detected by the human eye is actually only a very small part of the complete electromagnetic spectrum, which can be re garded as a continuum from the very low energies and long wavelengths characteristic ofradio waves to the very high energies (and shortwavelengths) of gamma rays and cosmic rays. As shown in Figure 4.1, the more familiar regions ofthe infrared, visible light, ultraviolet. and X-rays fall between these extremes of energy and wavelength. Points in the electromagnetic spectrum can be specified using a variety ofenergy or wavelength units. The most com mon energy unit employed by physicists is the electron volt' (eV). -
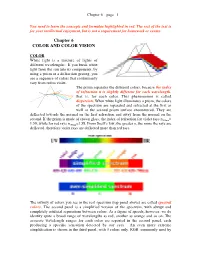
Chapter 6 COLOR and COLOR VISION
Chapter 6 – page 1 You need to learn the concepts and formulae highlighted in red. The rest of the text is for your intellectual enjoyment, but is not a requirement for homework or exams. Chapter 6 COLOR AND COLOR VISION COLOR White light is a mixture of lights of different wavelengths. If you break white light from the sun into its components, by using a prism or a diffraction grating, you see a sequence of colors that continuously vary from red to violet. The prism separates the different colors, because the index of refraction n is slightly different for each wavelength, that is, for each color. This phenomenon is called dispersion. When white light illuminates a prism, the colors of the spectrum are separated and refracted at the first as well as the second prism surface encountered. They are deflected towards the normal on the first refraction and away from the normal on the second. If the prism is made of crown glass, the index of refraction for violet rays n400nm= 1.59, while for red rays n700nm=1.58. From Snell’s law, the greater n, the more the rays are deflected, therefore violet rays are deflected more than red rays. The infinity of colors you see in the real spectrum (top panel above) are called spectral colors. The second panel is a simplified version of the spectrum, with abrupt and completely artificial separations between colors. As a figure of speech, however, we do identify quite a broad range of wavelengths as red, another as orange and so on. -

Optical Properties of Tio2 Based Multilayer Thin Films: Application to Optical Filters
Int. J. Thin Fil. Sci. Tec. 4, No. 1, 17-21 (2015) 17 International Journal of Thin Films Science and Technology http://dx.doi.org/10.12785/ijtfst/040104 Optical Properties of TiO2 Based Multilayer Thin Films: Application to Optical Filters. M. Kitui1, M. M Mwamburi2, F. Gaitho1, C. M. Maghanga3,*. 1Department of Physics, Masinde Muliro University of Science and Technology, P.O. Box 190, 50100, Kakamega, Kenya. 2Department of Physics, University of Eldoret, P.O. Box 1125 Eldoret, Kenya. 3Department of computer and Mathematics, Kabarak University, P.O. Private Bag Kabarak, Kenya. Received: 6 Jul. 2014, Revised: 13 Oct. 2014, Accepted: 19 Oct. 2014. Published online: 1 Jan. 2015. Abstract: Optical filters have received much attention currently due to the increasing demand in various applications. Spectral filters block specific wavelengths or ranges of wavelengths and transmit the rest of the spectrum. This paper reports on the simulated TiO2 – SiO2 optical filters. The design utilizes a high refractive index TiO2 thin films which were fabricated using spray Pyrolysis technique and low refractive index SiO2 obtained theoretically. The refractive index and extinction coefficient of the fabricated TiO2 thin films were extracted by simulation based on the best fit. This data was then used to design a five alternating layer stack which resulted into band pass with notch filters. The number of band passes and notches increase with the increase of individual layer thickness in the stack. Keywords: Multilayer, Optical filter, TiO2 and SiO2, modeling. 1. Introduction successive boundaries of different layers of the stack. The interface formed between the alternating layers has a great influence on the performance of the multilayer devices [4]. -
Computer Graphicsgraphics -- Weekweek 1212
ComputerComputer GraphicsGraphics -- WeekWeek 1212 Bengt-Olaf Schneider IBM T.J. Watson Research Center Questions about Last Week ? Computer Graphics – Week 12 © Bengt-Olaf Schneider, 1999 Overview of Week 12 Graphics Hardware Output devices (CRT and LCD) Graphics architectures Performance modeling Color Color theory Color gamuts Gamut matching Color spaces (next week) Computer Graphics – Week 12 © Bengt-Olaf Schneider, 1999 Graphics Hardware: Overview Display technologies Graphics architecture fundamentals Many of the general techniques discussed earlier in the semester were developed with hardware in mind Close similarity between software and hardware architectures Computer Graphics – Week 12 © Bengt-Olaf Schneider, 1999 Display Technologies CRT (Cathode Ray Tube) LCD (Liquid Crystal Display) Computer Graphics – Week 12 © Bengt-Olaf Schneider, 1999 CRT: Basic Structure (1) Top View Vertical Deflection Collector Control Grid System Electrode Phosphor Electron Beam Cathode Electron Lens Horizontal Deflection System Computer Graphics – Week 12 © Bengt-Olaf Schneider, 1999 CRT: Basic Structure (2) Deflection System Typically magnetic and not electrostatic Reduces the length of the tube and allows wider Vertical Deflection Collector deflection angles Control Grid System Electrode Phosphor Phospor Electron Beam Cathode Electron Lens Horizontal Fluorescence: Light emitted Deflection System after impact of electrons Persistence: Duration of emission after beam is turned off. Determines flicker properties of the CRT. Computer Graphics – Week -

About SI Units
Units SI units: International system of units (French: “SI” means “Système Internationale”) The seven SI base units are: Mass: kg Defined by the prototype “standard kilogram” located in Paris. The standard kilogram is made of Pt metal. Length: m Originally defined by the prototype “standard meter” located in Paris. Then defined as 1,650,763.73 wavelengths of the orange-red radiation of Krypton86 under certain specified conditions. (Official definition: The distance traveled by light in vacuum during a time interval of 1 / 299 792 458 of a second) Time: s The second is defined as the duration of a certain number of oscillations of radiation coming from Cs atoms. (Official definition: The second is the duration of 9,192,631,770 periods of the radiation of the hyperfine- level transition of the ground state of the Cs133 atom) Current: A Defined as the current that causes a certain force between two parallel wires. (Official definition: The ampere is that constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross-section, and placed 1 meter apart in vacuum, would produce between these conductors a force equal to 2 × 10-7 Newton per meter of length. Temperature: K One percent of the temperature difference between boiling point and freezing point of water. (Official definition: The Kelvin, unit of thermodynamic temperature, is the fraction 1 / 273.16 of the thermodynamic temperature of the triple point of water. Amount of substance: mol The amount of a substance that contains Avogadro’s number NAvo = 6.0221 × 1023 of atoms or molecules. -

Black Body Radiation and Radiometric Parameters
Black Body Radiation and Radiometric Parameters: All materials absorb and emit radiation to some extent. A blackbody is an idealization of how materials emit and absorb radiation. It can be used as a reference for real source properties. An ideal blackbody absorbs all incident radiation and does not reflect. This is true at all wavelengths and angles of incidence. Thermodynamic principals dictates that the BB must also radiate at all ’s and angles. The basic properties of a BB can be summarized as: 1. Perfect absorber/emitter at all ’s and angles of emission/incidence. Cavity BB 2. The total radiant energy emitted is only a function of the BB temperature. 3. Emits the maximum possible radiant energy from a body at a given temperature. 4. The BB radiation field does not depend on the shape of the cavity. The radiation field must be homogeneous and isotropic. T If the radiation going from a BB of one shape to another (both at the same T) were different it would cause a cooling or heating of one or the other cavity. This would violate the 1st Law of Thermodynamics. T T A B Radiometric Parameters: 1. Solid Angle dA d r 2 where dA is the surface area of a segment of a sphere surrounding a point. r d A r is the distance from the point on the source to the sphere. The solid angle looks like a cone with a spherical cap. z r d r r sind y r sin x An element of area of a sphere 2 dA rsin d d Therefore dd sin d The full solid angle surrounding a point source is: 2 dd sind 00 2cos 0 4 Or integrating to other angles < : 21cos The unit of solid angle is steradian. -

Section 22-3: Energy, Momentum and Radiation Pressure
Answer to Essential Question 22.2: (a) To find the wavelength, we can combine the equation with the fact that the speed of light in air is 3.00 " 108 m/s. Thus, a frequency of 1 " 1018 Hz corresponds to a wavelength of 3 " 10-10 m, while a frequency of 90.9 MHz corresponds to a wavelength of 3.30 m. (b) Using Equation 22.2, with c = 3.00 " 108 m/s, gives an amplitude of . 22-3 Energy, Momentum and Radiation Pressure All waves carry energy, and electromagnetic waves are no exception. We often characterize the energy carried by a wave in terms of its intensity, which is the power per unit area. At a particular point in space that the wave is moving past, the intensity varies as the electric and magnetic fields at the point oscillate. It is generally most useful to focus on the average intensity, which is given by: . (Eq. 22.3: The average intensity in an EM wave) Note that Equations 22.2 and 22.3 can be combined, so the average intensity can be calculated using only the amplitude of the electric field or only the amplitude of the magnetic field. Momentum and radiation pressure As we will discuss later in the book, there is no mass associated with light, or with any EM wave. Despite this, an electromagnetic wave carries momentum. The momentum of an EM wave is the energy carried by the wave divided by the speed of light. If an EM wave is absorbed by an object, or it reflects from an object, the wave will transfer momentum to the object. -

Environmental Impacts of Photoluminescence and Light
sustainability Article Environmental Impacts of Photoluminescence and Light-Emitting Diode (LED) Lighting Technologies in Horticulture: Case Study on Compact Fluorescent Lamp (CFL) and LED Lights for “Night Break” of Chrysanthemum Cultivation Thi Thu Linh Hoang 1,2,*, Thi Gam Do 1, Van Thao Nguyen 1, Hoai Chau Nguyen 2,3 and Hong Khoi Phan 1,* 1 Center for High Technology Development, VAST, Hanoi 10000, Vietnam; [email protected] (T.G.D.); [email protected] (V.T.N.) 2 Faculty of Environmental Technology, Graduate University of Science & Technology (GUST), VAST, Hanoi 10000, Vietnam; [email protected] 3 Institute of Environmental Technology (IET), VAST, Hanoi 10000, Vietnam * Correspondence: [email protected] (T.T.L.H.); [email protected] (H.K.P.); Tel.: +84-98-3416689 (T.T.L.H.); Fax: +84-24-3791-6283 (T.T.L.H.) Received: 7 August 2020; Accepted: 17 September 2020; Published: 25 September 2020 Abstract: The environmental impacts of photoluminescence and light-emitting diode (LED) lighting technologies in horticulture are described in this paper. As a case study, the life cycle assessment (LCA) associated with the raw materials, air, water and natural resources of screw-based compact fluorescent lamps (CFLs) and screw-based horticultural LED lamps (abbreviated as H-LED) used for “night break” effect in chrysanthemum cultivation is considered. Instead of the unit of radiant power of lighting sources (lumens) used in human lighting, the photon flux (micromoles per second) of the plant light is used in this study. The results of the study show that the environmental impacts of the H-LED lighting technology are markedly less than the fluorescent lamps.