International System of Units (Si)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Luminance Requirements for Lighted Signage
Luminance Requirements for Lighted Signage Jean Paul Freyssinier, Nadarajah Narendran, John D. Bullough Lighting Research Center Rensselaer Polytechnic Institute, Troy, NY 12180 www.lrc.rpi.edu Freyssinier, J.P., N. Narendran, and J.D. Bullough. 2006. Luminance requirements for lighted signage. Sixth International Conference on Solid State Lighting, Proceedings of SPIE 6337, 63371M. Copyright 2006 Society of Photo-Optical Instrumentation Engineers. This paper was published in the Sixth International Conference on Solid State Lighting, Proceedings of SPIE and is made available as an electronic preprint with permission of SPIE. One print or electronic copy may be made for personal use only. Systematic or multiple reproduction, distribution to multiple locations via electronic or other means, duplication of any material in this paper for a fee or for commercial purposes, or modification of the content of the paper are prohibited. Luminance Requirements for Lighted Signage Jean Paul Freyssinier*, Nadarajah Narendran, John D. Bullough Lighting Research Center, Rensselaer Polytechnic Institute, 21 Union Street, Troy, NY 12180 USA ABSTRACT Light-emitting diode (LED) technology is presently targeted to displace traditional light sources in backlighted signage. The literature shows that brightness and contrast are perhaps the two most important elements of a sign that determine its attention-getting capabilities and its legibility. Presently, there are no luminance standards for signage, and the practice of developing brighter signs to compete with signs in adjacent businesses is becoming more commonplace. Sign luminances in such cases may far exceed what people usually need for identifying and reading a sign. Furthermore, the practice of higher sign luminance than needed has many negative consequences, including higher energy use and light pollution. -

Report on Digital Sign Brightness
REPORT ON DIGITAL SIGN BRIGHTNESS Prepared for the Nevada State Department of Transportation, Washoe County, City of Reno and City of Sparks By Jerry Wachtel, President, The Veridian Group, Inc., Berkeley, CA November 2014 1 Table of Contents PART 1 ......................................................................................................................... 3 Introduction. ................................................................................................................ 3 Background. ................................................................................................................. 3 Key Terms and Definitions. .......................................................................................... 4 Luminance. .................................................................................................................................................................... 4 Illuminance. .................................................................................................................................................................. 5 Reflected Light vs. Emitted Light (Traditional Signs vs. Electronic Signs). ...................................... 5 Measuring Luminance and Illuminance ........................................................................ 6 Measuring Luminance. ............................................................................................................................................. 6 Measuring Illuminance. .......................................................................................................................................... -

Lecture 3: the Sensor
4.430 Daylighting Human Eye ‘HDR the old fashioned way’ (Niemasz) Massachusetts Institute of Technology ChriChristoph RstophReeiinhartnhart Department of Architecture 4.4.430 The430The SeSensnsoror Building Technology Program Happy Valentine’s Day Sun Shining on a Praline Box on February 14th at 9.30 AM in Boston. 1 Happy Valentine’s Day Falsecolor luminance map Light and Human Vision 2 Human Eye Outside view of a human eye Ophtalmogram of a human retina Retina has three types of photoreceptors: Cones, Rods and Ganglion Cells Day and Night Vision Photopic (DaytimeVision): The cones of the eye are of three different types representing the three primary colors, red, green and blue (>3 cd/m2). Scotopic (Night Vision): The rods are repsonsible for night and peripheral vision (< 0.001 cd/m2). Mesopic (Dim Light Vision): occurs when the light levels are low but one can still see color (between 0.001 and 3 cd/m2). 3 VisibleRange Daylighting Hanbook (Reinhart) The human eye can see across twelve orders of magnitude. We can adapt to about 10 orders of magnitude at a time via the iris. Larger ranges take time and require ‘neural adaptation’. Transition Spaces Outside Atrium Circulation Area Final destination 4 Luminous Response Curve of the Human Eye What is daylight? Daylight is the visible part of the electromagnetic spectrum that lies between 380 and 780 nm. UV blue green yellow orange red IR 380 450 500 550 600 650 700 750 wave length (nm) 5 Photometric Quantities Characterize how a space is perceived. Illuminance Luminous Flux Luminance Luminous Intensity Luminous Intensity [Candela] ~ 1 candela Courtesy of Matthew Bowden at www.digitallyrefreshing.com. -

Compton Scattering from the Deuteron at Low Energies
SE0200264 LUNFD6-NFFR-1018 Compton Scattering from the Deuteron at Low Energies Magnus Lundin Department of Physics Lund University 2002 W' •sii" Compton spridning från deuteronen vid låga energier (populärvetenskaplig sammanfattning på svenska) Vid Compton spridning sprids fotonen elastiskt, dvs. utan att förlora energi, mot en annan partikel eller kärna. Kärnorna som användes i detta försök består av en proton och en neutron (sk. deuterium, eller tungt väte). Kärnorna bestrålades med fotoner av kända energier och de spridda fo- tonerna detekterades mha. stora Nal-detektorer som var placerade i olika vinklar runt strålmålet. Försöket utfördes under 8 veckor och genom att räkna antalet fotoner som kärnorna bestålades med och antalet spridda fo- toner i de olika detektorerna, kan sannolikheten för att en foton skall spridas bestämmas. Denna sannolikhet jämfördes med en teoretisk modell som beskriver sannolikheten för att en foton skall spridas elastiskt mot en deuterium- kärna. Eftersom protonen och neutronen består av kvarkar, vilka har en elektrisk laddning, kommer dessa att sträckas ut då de utsätts för ett elek- triskt fält (fotonen), dvs. de polariseras. Värdet (sannolikheten) som den teoretiska modellen ger, beror på polariserbarheten hos protonen och neu- tronen i deuterium. Genom att beräkna sannolikheten för fotonspridning för olika värden av polariserbarheterna, kan man se vilket värde som ger bäst överensstämmelse mellan modellen och experimentella data. Det är speciellt neutronens polariserbarhet som är av intresse, och denna kunde bestämmas i detta arbete. Organization Document name LUND UNIVERSITY DOCTORAL DISSERTATION Department of Physics Date of issue 2002.04.29 Division of Nuclear Physics Box 118 Sponsoring organization SE-22100 Lund Sweden Author (s) Magnus Lundin Title and subtitle Compton Scattering from the Deuteron at Low Energies Abstract A series of three Compton scattering experiments on deuterium have been performed at the high-resolution tagged-photon facility MAX-lab located in Lund, Sweden. -

Fundametals of Rendering - Radiometry / Photometry
Fundametals of Rendering - Radiometry / Photometry “Physically Based Rendering” by Pharr & Humphreys •Chapter 5: Color and Radiometry •Chapter 6: Camera Models - we won’t cover this in class 782 Realistic Rendering • Determination of Intensity • Mechanisms – Emittance (+) – Absorption (-) – Scattering (+) (single vs. multiple) • Cameras or retinas record quantity of light 782 Pertinent Questions • Nature of light and how it is: – Measured – Characterized / recorded • (local) reflection of light • (global) spatial distribution of light 782 Electromagnetic spectrum 782 Spectral Power Distributions e.g., Fluorescent Lamps 782 Tristimulus Theory of Color Metamers: SPDs that appear the same visually Color matching functions of standard human observer International Commision on Illumination, or CIE, of 1931 “These color matching functions are the amounts of three standard monochromatic primaries needed to match the monochromatic test primary at the wavelength shown on the horizontal scale.” from Wikipedia “CIE 1931 Color Space” 782 Optics Three views •Geometrical or ray – Traditional graphics – Reflection, refraction – Optical system design •Physical or wave – Dispersion, interference – Interaction of objects of size comparable to wavelength •Quantum or photon optics – Interaction of light with atoms and molecules 782 What Is Light ? • Light - particle model (Newton) – Light travels in straight lines – Light can travel through a vacuum (waves need a medium to travel in) – Quantum amount of energy • Light – wave model (Huygens): electromagnetic radiation: sinusiodal wave formed coupled electric (E) and magnetic (H) fields 782 Nature of Light • Wave-particle duality – Light has some wave properties: frequency, phase, orientation – Light has some quantum particle properties: quantum packets (photons). • Dimensions of light – Amplitude or Intensity – Frequency – Phase – Polarization 782 Nature of Light • Coherence - Refers to frequencies of waves • Laser light waves have uniform frequency • Natural light is incoherent- waves are multiple frequencies, and random in phase. -

Light and Illumination
ChapterChapter 3333 -- LightLight andand IlluminationIllumination AAA PowerPointPowerPointPowerPoint PresentationPresentationPresentation bybyby PaulPaulPaul E.E.E. Tippens,Tippens,Tippens, ProfessorProfessorProfessor ofofof PhysicsPhysicsPhysics SouthernSouthernSouthern PolytechnicPolytechnicPolytechnic StateStateState UniversityUniversityUniversity © 2007 Objectives:Objectives: AfterAfter completingcompleting thisthis module,module, youyou shouldshould bebe ableable to:to: •• DefineDefine lightlight,, discussdiscuss itsits properties,properties, andand givegive thethe rangerange ofof wavelengthswavelengths forfor visiblevisible spectrum.spectrum. •• ApplyApply thethe relationshiprelationship betweenbetween frequenciesfrequencies andand wavelengthswavelengths forfor opticaloptical waves.waves. •• DefineDefine andand applyapply thethe conceptsconcepts ofof luminousluminous fluxflux,, luminousluminous intensityintensity,, andand illuminationillumination.. •• SolveSolve problemsproblems similarsimilar toto thosethose presentedpresented inin thisthis module.module. AA BeginningBeginning DefinitionDefinition AllAll objectsobjects areare emittingemitting andand absorbingabsorbing EMEM radiaradia-- tiontion.. ConsiderConsider aa pokerpoker placedplaced inin aa fire.fire. AsAs heatingheating occurs,occurs, thethe 1 emittedemitted EMEM waveswaves havehave 2 higherhigher energyenergy andand 3 eventuallyeventually becomebecome visible.visible. 4 FirstFirst redred .. .. .. thenthen white.white. LightLightLight maymaymay bebebe defineddefineddefined -

About SI Units
Units SI units: International system of units (French: “SI” means “Système Internationale”) The seven SI base units are: Mass: kg Defined by the prototype “standard kilogram” located in Paris. The standard kilogram is made of Pt metal. Length: m Originally defined by the prototype “standard meter” located in Paris. Then defined as 1,650,763.73 wavelengths of the orange-red radiation of Krypton86 under certain specified conditions. (Official definition: The distance traveled by light in vacuum during a time interval of 1 / 299 792 458 of a second) Time: s The second is defined as the duration of a certain number of oscillations of radiation coming from Cs atoms. (Official definition: The second is the duration of 9,192,631,770 periods of the radiation of the hyperfine- level transition of the ground state of the Cs133 atom) Current: A Defined as the current that causes a certain force between two parallel wires. (Official definition: The ampere is that constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross-section, and placed 1 meter apart in vacuum, would produce between these conductors a force equal to 2 × 10-7 Newton per meter of length. Temperature: K One percent of the temperature difference between boiling point and freezing point of water. (Official definition: The Kelvin, unit of thermodynamic temperature, is the fraction 1 / 273.16 of the thermodynamic temperature of the triple point of water. Amount of substance: mol The amount of a substance that contains Avogadro’s number NAvo = 6.0221 × 1023 of atoms or molecules. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Exposure Metering and Zone System Calibration
Exposure Metering Relating Subject Lighting to Film Exposure By Jeff Conrad A photographic exposure meter measures subject lighting and indicates camera settings that nominally result in the best exposure of the film. The meter calibration establishes the relationship between subject lighting and those camera settings; the photographer’s skill and metering technique determine whether the camera settings ultimately produce a satisfactory image. Historically, the “best” exposure was determined subjectively by examining many photographs of different types of scenes with different lighting levels. Common practice was to use wide-angle averaging reflected-light meters, and it was found that setting the calibration to render the average of scene luminance as a medium tone resulted in the “best” exposure for many situations. Current calibration standards continue that practice, although wide-angle average metering largely has given way to other metering tech- niques. In most cases, an incident-light meter will cause a medium tone to be rendered as a medium tone, and a reflected-light meter will cause whatever is metered to be rendered as a medium tone. What constitutes a “medium tone” depends on many factors, including film processing, image postprocessing, and, when appropriate, the printing process. More often than not, a “medium tone” will not exactly match the original medium tone in the subject. In many cases, an exact match isn’t necessary—unless the original subject is available for direct comparison, the viewer of the image will be none the wiser. It’s often stated that meters are “calibrated to an 18% reflectance,” usually without much thought given to what the statement means. -
Spectral Light Measuring
Spectral Light Measuring 1 Precision GOSSEN Foto- und Lichtmesstechnik – Your Guarantee for Precision and Quality GOSSEN Foto- und Lichtmesstechnik is specialized in the measurement of light, and has decades of experience in its chosen field. Continuous innovation is the answer to rapidly changing technologies, regulations and markets. Outstanding product quality is assured by means of a certified quality management system in accordance with ISO 9001. LED – Light of the Future The GOSSEN Light Lab LED technology has experience rapid growth in recent years thanks to the offers calibration services, for our own products, as well as for products from development of LEDs with very high light efficiency. This is being pushed by other manufacturers, and issues factory calibration certificates. The optical the ban on conventional light bulbs with low energy efficiency, as well as an table used for this purpose is subject to strict test equipment monitoring, and ever increasing energy-saving mentality and environmental awareness. LEDs is traced back to the PTB in Braunschweig, Germany (German Federal Institute have long since gone beyond their previous status as effects lighting and are of Physics and Metrology). Aside from the PTB, our lab is the first in Germany being used for display illumination, LED displays and lamps. Modern means to be accredited for illuminance by DAkkS (German accreditation authority), of transportation, signal systems and street lights, as well as indoor and and is thus authorized to issue internationally recognized DAkkS calibration outdoor lighting, are no longer conceivable without them. The brightness and certificates. This assures that acquired measured values comply with official color of LEDs vary due to manufacturing processes, for which reason they regulations and, as a rule, stand up to legal argumentation. -
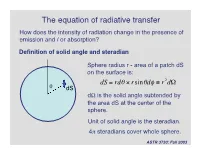
The Equation of Radiative Transfer How Does the Intensity of Radiation Change in the Presence of Emission and / Or Absorption?
The equation of radiative transfer How does the intensity of radiation change in the presence of emission and / or absorption? Definition of solid angle and steradian Sphere radius r - area of a patch dS on the surface is: dS = rdq ¥ rsinqdf ≡ r2dW q dS dW is the solid angle subtended by the area dS at the center of the † sphere. Unit of solid angle is the steradian. 4p steradians cover whole sphere. ASTR 3730: Fall 2003 Definition of the specific intensity Construct an area dA normal to a light ray, and consider all the rays that pass through dA whose directions lie within a small solid angle dW. Solid angle dW dA The amount of energy passing through dA and into dW in time dt in frequency range dn is: dE = In dAdtdndW Specific intensity of the radiation. † ASTR 3730: Fall 2003 Compare with definition of the flux: specific intensity is very similar except it depends upon direction and frequency as well as location. Units of specific intensity are: erg s-1 cm-2 Hz-1 steradian-1 Same as Fn Another, more intuitive name for the specific intensity is brightness. ASTR 3730: Fall 2003 Simple relation between the flux and the specific intensity: Consider a small area dA, with light rays passing through it at all angles to the normal to the surface n: n o In If q = 90 , then light rays in that direction contribute zero net flux through area dA. q For rays at angle q, foreshortening reduces the effective area by a factor of cos(q). -

4.1 – Radian and Degree Measure
4.1 { Radian and Degree Measure Accelerated Pre-Calculus Mr. Niedert Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 1 / 27 2 Radian Measure 3 Degree Measure 4 Applications 4.1 { Radian and Degree Measure 1 Angles Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 2 / 27 3 Degree Measure 4 Applications 4.1 { Radian and Degree Measure 1 Angles 2 Radian Measure Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 2 / 27 4 Applications 4.1 { Radian and Degree Measure 1 Angles 2 Radian Measure 3 Degree Measure Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 2 / 27 4.1 { Radian and Degree Measure 1 Angles 2 Radian Measure 3 Degree Measure 4 Applications Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 2 / 27 The starting position of the ray is the initial side of the angle. The position after the rotation is the terminal side of the angle. The vertex of the angle is the endpoint of the ray. Angles An angle is determine by rotating a ray about it endpoint. Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 3 / 27 The position after the rotation is the terminal side of the angle. The vertex of the angle is the endpoint of the ray. Angles An angle is determine by rotating a ray about it endpoint. The starting position of the ray is the initial side of the angle. Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 3 / 27 The vertex of the angle is the endpoint of the ray.