Draft 9Th Edition of the SI Brochure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

4.1 – Radian and Degree Measure
4.1 { Radian and Degree Measure Accelerated Pre-Calculus Mr. Niedert Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 1 / 27 2 Radian Measure 3 Degree Measure 4 Applications 4.1 { Radian and Degree Measure 1 Angles Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 2 / 27 3 Degree Measure 4 Applications 4.1 { Radian and Degree Measure 1 Angles 2 Radian Measure Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 2 / 27 4 Applications 4.1 { Radian and Degree Measure 1 Angles 2 Radian Measure 3 Degree Measure Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 2 / 27 4.1 { Radian and Degree Measure 1 Angles 2 Radian Measure 3 Degree Measure 4 Applications Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 2 / 27 The starting position of the ray is the initial side of the angle. The position after the rotation is the terminal side of the angle. The vertex of the angle is the endpoint of the ray. Angles An angle is determine by rotating a ray about it endpoint. Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 3 / 27 The position after the rotation is the terminal side of the angle. The vertex of the angle is the endpoint of the ray. Angles An angle is determine by rotating a ray about it endpoint. The starting position of the ray is the initial side of the angle. Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 3 / 27 The vertex of the angle is the endpoint of the ray. -

Relationships of the SI Derived Units with Special Names and Symbols and the SI Base Units
Relationships of the SI derived units with special names and symbols and the SI base units Derived units SI BASE UNITS without special SI DERIVED UNITS WITH SPECIAL NAMES AND SYMBOLS names Solid lines indicate multiplication, broken lines indicate division kilogram kg newton (kg·m/s2) pascal (N/m2) gray (J/kg) sievert (J/kg) 3 N Pa Gy Sv MASS m FORCE PRESSURE, ABSORBED DOSE VOLUME STRESS DOSE EQUIVALENT meter m 2 m joule (N·m) watt (J/s) becquerel (1/s) hertz (1/s) LENGTH J W Bq Hz AREA ENERGY, WORK, POWER, ACTIVITY FREQUENCY second QUANTITY OF HEAT HEAT FLOW RATE (OF A RADIONUCLIDE) s m/s TIME VELOCITY katal (mol/s) weber (V·s) henry (Wb/A) tesla (Wb/m2) kat Wb H T 2 mole m/s CATALYTIC MAGNETIC INDUCTANCE MAGNETIC mol ACTIVITY FLUX FLUX DENSITY ACCELERATION AMOUNT OF SUBSTANCE coulomb (A·s) volt (W/A) C V ampere A ELECTRIC POTENTIAL, CHARGE ELECTROMOTIVE ELECTRIC CURRENT FORCE degree (K) farad (C/V) ohm (V/A) siemens (1/W) kelvin Celsius °C F W S K CELSIUS CAPACITANCE RESISTANCE CONDUCTANCE THERMODYNAMIC TEMPERATURE TEMPERATURE t/°C = T /K – 273.15 candela 2 steradian radian cd lux (lm/m ) lumen (cd·sr) 2 2 (m/m = 1) lx lm sr (m /m = 1) rad LUMINOUS INTENSITY ILLUMINANCE LUMINOUS SOLID ANGLE PLANE ANGLE FLUX The diagram above shows graphically how the 22 SI derived units with special names and symbols are related to the seven SI base units. In the first column, the symbols of the SI base units are shown in rectangles, with the name of the unit shown toward the upper left of the rectangle and the name of the associated base quantity shown in italic type below the rectangle. -

The Kelvin and Temperature Measurements
Volume 106, Number 1, January–February 2001 Journal of Research of the National Institute of Standards and Technology [J. Res. Natl. Inst. Stand. Technol. 106, 105–149 (2001)] The Kelvin and Temperature Measurements Volume 106 Number 1 January–February 2001 B. W. Mangum, G. T. Furukawa, The International Temperature Scale of are available to the thermometry commu- K. G. Kreider, C. W. Meyer, D. C. 1990 (ITS-90) is defined from 0.65 K nity are described. Part II of the paper Ripple, G. F. Strouse, W. L. Tew, upwards to the highest temperature measur- describes the realization of temperature able by spectral radiation thermometry, above 1234.93 K for which the ITS-90 is M. R. Moldover, B. Carol Johnson, the radiation thermometry being based on defined in terms of the calibration of spec- H. W. Yoon, C. E. Gibson, and the Planck radiation law. When it was troradiometers using reference blackbody R. D. Saunders developed, the ITS-90 represented thermo- sources that are at the temperature of the dynamic temperatures as closely as pos- equilibrium liquid-solid phase transition National Institute of Standards and sible. Part I of this paper describes the real- of pure silver, gold, or copper. The realiza- Technology, ization of contact thermometry up to tion of temperature from absolute spec- 1234.93 K, the temperature range in which tral or total radiometry over the tempera- Gaithersburg, MD 20899-0001 the ITS-90 is defined in terms of calibra- ture range from about 60 K to 3000 K is [email protected] tion of thermometers at 15 fixed points and also described. -

The Kibble Balance and the Kilogram
C. R. Physique 20 (2019) 55–63 Contents lists available at ScienceDirect Comptes Rendus Physique www.sciencedirect.com The new International System of Units / Le nouveau Système international d’unités The Kibble balance and the kilogram La balance de Kibble et le kilogramme ∗ Stephan Schlamminger , Darine Haddad NIST, 100 Bureau Drive, Gaithersburg, MD 20899, USA a r t i c l e i n f o a b s t r a c t Article history: Dr. Bryan Kibble invented the watt balance in 1975 to improve the realization of the unit Available online 25 March 2019 for electrical current, the ampere. With the discovery of the Quantum Hall effect in 1980 by Dr. Klaus von Klitzing and in conjunction with the previously predicted Josephson effect, Keywords: this mechanical apparatus could be used to measure the Planck constant h. Following a Unit of mass proposal by Quinn, Mills, Williams, Taylor, and Mohr, the Kibble balance can be used to Kilogram Planck constant realize the unit of mass, the kilogram, by fixing the numerical value of Planck’s constant. Kibble balance In 2017, the watt balance was renamed to the Kibble balance to honor the inventor, who Revised SI passed in 2016. This article explains the Kibble balance, its role in the redefinition of the Josephson effect unit of mass, and attempts an outlook of the future. Quantum Hall effect Published by Elsevier Masson SAS on behalf of Académie des sciences. This is an open access article under the CC BY-NC-ND license Mots-clés : (http://creativecommons.org/licenses/by-nc-nd/4.0/). -

The International System of Units (SI)
NAT'L INST. OF STAND & TECH NIST National Institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 330 2001 Edition The International System of Units (SI) 4. Barry N. Taylor, Editor r A o o L57 330 2oOI rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -

SI Traceability and Scales for Underpinning Atmospheric
Metrologia PAPER Recent citations SI traceability and scales for underpinning - Amount of substance and the mole in the SI atmospheric monitoring of greenhouse gases Bernd Güttler et al - Advances in reference materials and To cite this article: Paul J Brewer et al 2018 Metrologia 55 S174 measurement techniques for greenhouse gas atmospheric observations Paul J Brewer et al - News from the BIPM laboratories—2018 Patrizia Tavella et al View the article online for updates and enhancements. This content was downloaded from IP address 205.156.36.134 on 08/07/2019 at 18:02 IOP Metrologia Bureau International des Poids et Mesures Metrologia Metrologia Metrologia 55 (2018) S174–SS181 https://doi.org/10.1088/1681-7575/aad830 55 SI traceability and scales for underpinning 2018 atmospheric monitoring of greenhouse © 2018 BIPM & IOP Publishing Ltd gases MTRGAU Paul J Brewer1,6 , Richard J C Brown1 , Oksana A Tarasova2, Brad Hall3, George C Rhoderick4 and Robert I Wielgosz5 S174 1 National Physical Laboratory, Hampton Road, Teddington, Middlesex TW11 0LW, United Kingdom 2 World Meteorological Organization, 7bis, avenue de la Paix, Case postale 2300, CH-1211 Geneva 2, Switzerland 3 National Oceanic and Atmospheric Administration, 325 Broadway, Mail Stop R.GMD1, Boulder, CO 80305, United States of America P J Brewer et al 4 National Institute of Standards and Technology, 100 Bureau Drive, MS-8393 Gaithersburg, MD 20899-8393, United States of America 5 Printed in the UK Bureau International des Poids et Mesures, Pavillon de Breteuil, F-92312 Sèvres Cedex, France E-mail: [email protected] MET Received 21 June 2018, revised 2 August 2018 Accepted for publication 6 August 2018 10.1088/1681-7575/aad830 Published 7 September 2018 Abstract Paper Metrological traceability is the property of a measurement result whereby it can be related to a stated reference through a documented unbroken chain of calibrations, each contributing to the measurement uncertainty. -

The Redefinition of the Ampere
56TH INTERNATIONAL SCIENTIFIC COLLOQUIUM URN (Paper): urn:nbn:de:gbv:ilm1-2011iwk-151:3 Ilmenau University of Technology, 12 – 16 September 2011 URN: urn:nbn:gbv:ilm1-2011iwk:5 THE REDEFINITION OF THE AMPERE Franz Josef Ahlers, Uwe Siegner Physikalisch-Technische Bundesanstalt, Bundesallee 100, 38116 Braunschweig ABSTRACT F/l = μ0·(I²/2πr) in vacuum. The numerical value of the magnetic constant μ0 is fixed through the -7 The ampere is one of the seven base units of the SI, definition of the ampere to μ0 = 4π·10 N/A² the international system of units. Its definition is Since the numerical value of the speed of light c linked to mechanical units, especially the unit of has been fixed through the definition of the meter, mass, the kilogram. In a future system of units, which also the numerical value of the electric constant ε0 is 2 will be based on the values of fundamental constants, fixed according to the Maxwell relation μ0·ε0·c = l. the ampere will be based on the value of the The practical implementation of this definition – elementary charge e. This paper describes the which, in metrology, is called realisation – must be technical background of the redefinition. performed with the aid of measuring arrangements that can be realised experimentally. One dis- Index Terms – SI units, Josephson effect, tinguishes between direct realisation – in which the quantum Hall effect, single electron effect current is related to a mechanical force – and indirect realisation. The latter is based on Ohm's law I = U/R and the ampere is realised through the realisation of 1. -

Angles Are Inherently Neither Length Ratios Nor Dimensionless
Angles are inherently neither length ratios nor dimensionless Paul Quinceya, Peter J Mohrb and William D Phillipsb a National Physical Laboratory, Hampton Road, Teddington, TW11 0LW, United Kingdom b National Institute of Standards and Technology, Gaithersburg, MD 20899, USA Accepted for publication by Metrologia, 7 June 2019 Abstract The status of angles within The International System of Units (SI) has long been a source of controversy and confusion. We address one specific but crucial issue, putting the case that the idea of angles necessarily being length ratios, and hence dimensionless, is not valid. By making a clear distinction between the definition of a quantity and the process of making a measurement of that quantity, we show that the usual arguments for angles being length ratios are problematic. An appreciation of this point should clear away a major obstacle preventing a more proper treatment of the angle problem. Introduction One way to point out the odd status of angles within the SI is as follows: angle is measurable with a scale, on a protractor, in analogy to the way length is measureable with a ruler. It can therefore have units that are chosen by the user. This simple point is reinforced by the fact that multiple units for angle are used in practice. Angle measurements often use the degree, while the radian, which is used in mathematics and theoretical physics, is the SI unit. Other units, such as the grad, are used in specialised areas. And yet angle is treated as being dimensionless in the SI, a status usually applied only to quantities that are simply counted, like the number of molecules in a container, or quantities that are clearly a ratio of two values of the same quantity, such as refractive index. -

The New Mise En Pratique for the Metre—A Review of Approaches for the Practical Realization of Traceable Length Metrology from 10−11 M to 1013 M
Metrologia REVIEW • OPEN ACCESS The new mise en pratique for the metre—a review of approaches for the practical realization of traceable length metrology from 10−11 m to 1013 m To cite this article: René Schödel et al 2021 Metrologia 58 052002 View the article online for updates and enhancements. This content was downloaded from IP address 193.60.240.99 on 18/08/2021 at 10:04 Bureau International des Poids et Mesures Metrologia Metrologia 58 (2021) 052002 (20pp) https://doi.org/10.1088/1681-7575/ac1456 Review The new mise en pratique for the metre—a review of approaches for the practical realization of traceable length metrology from 10−11 mto1013 m Rene´ Schödel1,∗ , Andrew Yacoot2 and Andrew Lewis2 1 Physikalisch-Technische Bundesanstalt (PTB), Bundesallee 100, 38116 Braunschweig, Germany 2 National Physical Laboratory (NPL), Hampton Road, Teddington, Middlesex, TW11 0LW, United Kingdom E-mail: [email protected] Received 9 April 2021, revised 7 July 2021 Accepted for publication 14 July 2021 Published 5 August 2021 Abstract The revised International System of Units (SI) came into force on May 20, 2019. Simultaneously, updated versions of supporting documents for the practical realization of the SI base units (mises en pratique) were published. This review paper provides an overview of the updated mise en pratique for the SI base unit of length, the metre, that now gives practical guidance on realisation of traceable length metrology spanning 24 orders of magnitude. The review begins by showing how the metre may be primarily realized through time of flight and interferometric techniques using a variety of types of interferometer. -

Recommendation of a Consensus Value of the Ozone Absorption Cross-Section at 253.65 Nm Based on a Literature Review J
Recommendation of a consensus value of the ozone absorption cross-section at 253.65 nm based on a literature review J. Hodges, J. Viallon, P. Brewer, B. Drouin, V. Gorshelev, C. Janssen, S. Lee, A. Possolo, M. Smith, J. Walden, et al. To cite this version: J. Hodges, J. Viallon, P. Brewer, B. Drouin, V. Gorshelev, et al.. Recommendation of a consensus value of the ozone absorption cross-section at 253.65 nm based on a literature review. Metrologia, IOP Publishing, 2019, 56 (3), pp.034001. 10.1088/1681-7575/ab0bdd. hal-02108262 HAL Id: hal-02108262 https://hal.sorbonne-universite.fr/hal-02108262 Submitted on 24 Apr 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Metrologia PAPER • OPEN ACCESS Recommendation of a consensus value of the ozone absorption cross- section at 253.65 nm based on a literature review To cite this article: J T Hodges et al 2019 Metrologia 56 034001 View the article online for updates and enhancements. This content was downloaded from IP address 134.157.80.196 on 24/04/2019 at 08:46 IOP Metrologia Metrologia Metrologia Metrologia -
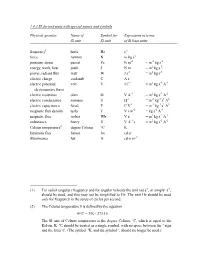
1.4.3 SI Derived Units with Special Names and Symbols
1.4.3 SI derived units with special names and symbols Physical quantity Name of Symbol for Expression in terms SI unit SI unit of SI base units frequency1 hertz Hz s-1 force newton N m kg s-2 pressure, stress pascal Pa N m-2 = m-1 kg s-2 energy, work, heat joule J N m = m2 kg s-2 power, radiant flux watt W J s-1 = m2 kg s-3 electric charge coulomb C A s electric potential, volt V J C-1 = m2 kg s-3 A-1 electromotive force electric resistance ohm Ω V A-1 = m2 kg s-3 A-2 electric conductance siemens S Ω-1 = m-2 kg-1 s3 A2 electric capacitance farad F C V-1 = m-2 kg-1 s4 A2 magnetic flux density tesla T V s m-2 = kg s-2 A-1 magnetic flux weber Wb V s = m2 kg s-2 A-1 inductance henry H V A-1 s = m2 kg s-2 A-2 Celsius temperature2 degree Celsius °C K luminous flux lumen lm cd sr illuminance lux lx cd sr m-2 (1) For radial (angular) frequency and for angular velocity the unit rad s-1, or simply s-1, should be used, and this may not be simplified to Hz. The unit Hz should be used only for frequency in the sense of cycles per second. (2) The Celsius temperature θ is defined by the equation θ/°C = T/K - 273.15 The SI unit of Celsius temperature is the degree Celsius, °C, which is equal to the Kelvin, K.