Intensity Wave Length
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
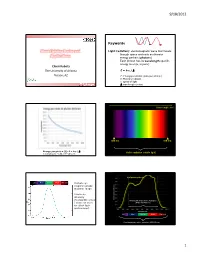
Plant Lighting Basics and Applications
9/18/2012 Keywords Plant Lighting Basics and Light (radiation): electromagnetic wave that travels through space and exits as discrete Applications energy packets (photons) Each photon has its wavelength-specific energy level (E, in joule) Chieri Kubota The University of Arizona E = h·c / Tucson, AZ E: Energy per photon (joule per photon) h: Planck’s constant c: speed of light Greensys 2011, Greece : wavelength (meter) Wavelength (nm) 380 nm 780 nm Energy per photon [J]: E = h·c / Visible radiation (visible light) mole photons = 6.02 x 1023 photons Leaf photosynthesis UV Blue Green Red Far red Human eye response peaks at green range. Luminous intensity (footcandle or lux Photosynthetically Active Radiation ) does not work (PAR, 400-700 nm) for plant light environment. UV Blue Green Red Far red Plant biologically active radiation (300-800 nm) 1 9/18/2012 Chlorophyll a Phytochrome response Chlorophyll b UV Blue Green Red Far red Absorption spectra of isolated chlorophyll Absorptance 400 500 600 700 Wavelength (nm) Daily light integral (DLI or Daily Light Unit and Terminology PPF) Radiation Photons Visible light •Total amount of photosynthetically active “Base” unit Energy (J) Photons (mol) Luminous intensity (cd) radiation (400‐700 nm) received per sq Flux [total amount received Radiant flux Photon flux Luminous flux meter per day or emitted per time] (J s-1) or (W) (mol s-1) (lm) •Unit: mole per sq meter per day (mol m‐2 d‐1) Flux density [total amount Radiant flux density Photon flux (density) Illuminance, • Under optimal conditions, plant growth is received per area per time] (W m-2) (mol m-2 s-1) Luminous flux density highly correlated with DLI. -

Measurement of the Earth Radiation Budget at the Top of the Atmosphere—A Review
Review Measurement of the Earth Radiation Budget at the Top of the Atmosphere—A Review Steven Dewitte * and Nicolas Clerbaux Observations Division, Royal Meteorological Institute of Belgium, 1180 Brussels, Belgium; [email protected] * Correspondence: [email protected]; Tel.: +32-2-3730624 Received: 25 September 2017; Accepted: 1 November 2017; Published: 7 November 2017 Abstract: The Earth Radiation Budget at the top of the atmosphere quantifies how the Earth gains energy from the Sun and loses energy to space. It is of fundamental importance for climate and climate change. In this paper, the current state-of-the-art of the satellite measurements of the Earth Radiation Budget is reviewed. Combining all available measurements, the most likely value of the Total Solar Irradiance at a solar minimum is 1362 W/m2, the most likely Earth albedo is 29.8%, and the most likely annual mean Outgoing Longwave Radiation is 238 W/m2. We highlight the link between long-term changes of the Outgoing Longwave Radiation, the strengthening of El Nino in the period 1985–1997 and the strengthening of La Nina in the period 2000–2009. Keywords: Earth Radiation Budget; Total Solar irradiance; Satellite remote sensing 1. Introduction The Earth Radiation Budget (ERB) at the top of the atmosphere describes how the Earth gains energy from the sun, and loses energy to space through reflection of solar radiation and the emission of thermal radiation. The ERB is of fundamental importance for climate since: (1) The global climate, as quantified e.g., by the global average temperature, is determined by this energy exchange. -

Chapter 2 Solar and Infrared Radiation Fluxes
Chapter 2 Solar and Infrared Radiation Chapter overview: • Fluxes • Energy transfer • Seasonal and daily changes in radiation • Surface radiation budget Fluxes Flux (F): The transfer of a quantity per unit area per unit time (sometimes called flux density). A flux can be thought of as the inflow or outflow of a quantity through the side of a fixed volume. Fluxes can occur in all three directions - Fx, Fy, and Fz What is the convention for the sign of a flux? We can consider fluxes of mass or of heat. What are the units for a mass flux or a heat flux? The amount of a quantity transferred through a given area (A) in a given time (Δt) can be calculated as: Amount = F ⋅ A⋅ Δt For a heat flux, the amount of heat transferred is represented by ΔQH. Note: The textbook discusses kinematic fluxes, but we will not discuss fluxes in these terms in ATOC 3050. Unlike the textbook, we will use the symbol F to represent fluxes, not kinematic fluxes. What processes can cause a heat flux? Radiant flux: The radiant energy per unit area per unit time. Radiant energy: Energy transferred by electromagnetic waves (radiation). Radiation emitted by the sun is referred to as solar or shortwave radiation. Shortwave radiation – refers to the wavelength band (< 4 µm) that carries most of the energy associated with solar radiation Solar constant (or total solar irradiance) (S0): The solar radiative flux, perpendicular to the solar beam, that enters the top of the atmosphere -2 S0 = 1366 W m Radiation emitted by the earth is referred to as longwave, terrestrial, or infrared radiation. -

Applied Spectroscopy Spectroscopic Nomenclature
Applied Spectroscopy Spectroscopic Nomenclature Absorbance, A Negative logarithm to the base 10 of the transmittance: A = –log10(T). (Not used: absorbancy, extinction, or optical density). (See Note 3). Absorptance, α Ratio of the radiant power absorbed by the sample to the incident radiant power; approximately equal to (1 – T). (See Notes 2 and 3). Absorption The absorption of electromagnetic radiation when light is transmitted through a medium; hence ‘‘absorption spectrum’’ or ‘‘absorption band’’. (Not used: ‘‘absorbance mode’’ or ‘‘absorbance band’’ or ‘‘absorbance spectrum’’ unless the ordinate axis of the spectrum is Absorbance.) (See Note 3). Absorption index, k See imaginary refractive index. Absorptivity, α Internal absorbance divided by the product of sample path length, ℓ , and mass concentration, ρ , of the absorbing material. A / α = i ρℓ SI unit: m2 kg–1. Common unit: cm2 g–1; L g–1 cm–1. (Not used: absorbancy index, extinction coefficient, or specific extinction.) Attenuated total reflection, ATR A sampling technique in which the evanescent wave of a beam that has been internally reflected from the internal surface of a material of high refractive index at an angle greater than the critical angle is absorbed by a sample that is held very close to the surface. (See Note 3.) Attenuation The loss of electromagnetic radiation caused by both absorption and scattering. Beer–Lambert law Absorptivity of a substance is constant with respect to changes in path length and concentration of the absorber. Often called Beer’s law when only changes in concentration are of interest. Brewster’s angle, θB The angle of incidence at which the reflection of p-polarized radiation is zero. -

Section 22-3: Energy, Momentum and Radiation Pressure
Answer to Essential Question 22.2: (a) To find the wavelength, we can combine the equation with the fact that the speed of light in air is 3.00 " 108 m/s. Thus, a frequency of 1 " 1018 Hz corresponds to a wavelength of 3 " 10-10 m, while a frequency of 90.9 MHz corresponds to a wavelength of 3.30 m. (b) Using Equation 22.2, with c = 3.00 " 108 m/s, gives an amplitude of . 22-3 Energy, Momentum and Radiation Pressure All waves carry energy, and electromagnetic waves are no exception. We often characterize the energy carried by a wave in terms of its intensity, which is the power per unit area. At a particular point in space that the wave is moving past, the intensity varies as the electric and magnetic fields at the point oscillate. It is generally most useful to focus on the average intensity, which is given by: . (Eq. 22.3: The average intensity in an EM wave) Note that Equations 22.2 and 22.3 can be combined, so the average intensity can be calculated using only the amplitude of the electric field or only the amplitude of the magnetic field. Momentum and radiation pressure As we will discuss later in the book, there is no mass associated with light, or with any EM wave. Despite this, an electromagnetic wave carries momentum. The momentum of an EM wave is the energy carried by the wave divided by the speed of light. If an EM wave is absorbed by an object, or it reflects from an object, the wave will transfer momentum to the object. -

CNT-Based Solar Thermal Coatings: Absorptance Vs. Emittance
coatings Article CNT-Based Solar Thermal Coatings: Absorptance vs. Emittance Yelena Vinetsky, Jyothi Jambu, Daniel Mandler * and Shlomo Magdassi * Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 9190401, Israel; [email protected] (Y.V.); [email protected] (J.J.) * Correspondence: [email protected] (D.M.); [email protected] (S.M.) Received: 15 October 2020; Accepted: 13 November 2020; Published: 17 November 2020 Abstract: A novel approach for fabricating selective absorbing coatings based on carbon nanotubes (CNTs) for mid-temperature solar–thermal application is presented. The developed formulations are dispersions of CNTs in water or solvents. Being coated on stainless steel (SS) by spraying, these formulations provide good characteristics of solar absorptance. The effect of CNT concentration and the type of the binder and its ratios to the CNT were investigated. Coatings based on water dispersions give higher adsorption, but solvent-based coatings enable achieving lower emittance. Interestingly, the binder was found to be responsible for the high emittance, yet, it is essential for obtaining good adhesion to the SS substrate. The best performance of the coatings requires adjusting the concentration of the CNTs and their ratio to the binder to obtain the highest absorptance with excellent adhesion; high absorptance is obtained at high CNT concentration, while good adhesion requires a minimum ratio between the binder/CNT; however, increasing the binder concentration increases the emissivity. The best coatings have an absorptance of ca. 90% with an emittance of ca. 0.3 and excellent adhesion to stainless steel. Keywords: carbon nanotubes (CNTs); binder; dispersion; solar thermal coating; absorptance; emittance; adhesion; selectivity 1. -

UNIT 1 ELECTROMAGNETIC RADIATION Radiation
Electromagnetic UNIT 1 ELECTROMAGNETIC RADIATION Radiation Structure 1.1 Introduction Objectives 1.2 What is Electromagnetic Radiation? Wave Mechanical Model of Electromagnetic Radiation Quantum Model of Electromagnetic Radiation 1.3 Consequences of Wave Nature of Electromagnetic Radiation Interference Diffraction Transmission Refraction Reflection Scattering Polarisation 1.4 Interaction of EM Radiation with Matter Absorption Emission Raman Scattering 1.5 Summary 1.6 Terminal Questions 1.7 Answers 1.1 INTRODUCTION You would surely have seen a beautiful rainbow showing seven different colours during the rainy season. You know that this colourful spectrum is due to the separation or dispersion of the white light into its constituent parts by the rain drops. The rainbow spectrum is just a minute part of a much larger continuum of the radiations that come from the sun. These are called electromagnetic radiations and the continuum of the electromagnetic radiations is called the electromagnetic spectrum. In the first unit of this course you would learn about the electromagnetic radiation in terms of its nature, characteristics and properties. Spectroscopy is the study of interaction of electromagnetic radiation with matter. We would discuss the ways in which different types of electromagnetic radiation interact with matter and also the types of spectra that result as a consequence of the interaction. In the next unit you would learn about ultraviolet-visible spectroscopy ‒ a consequence of interaction of electromagnetic radiation in the ultraviolet-visible -

Realization of a Micrometre-Sized Stochastic Heat Engine Valentin Blickle1,2* and Clemens Bechinger1,2
Erschienen in: Nature Physics ; 8 (2012), 2. - S. 143-146 https://dx.doi.org/10.1038/nphys2163 Realization of a micrometre-sized stochastic heat engine Valentin Blickle1,2* and Clemens Bechinger1,2 The conversion of energy into mechanical work is essential trapping centre and k(t), the time-dependent trap stiffness. The for almost any industrial process. The original description value of k is determined by the laser intensity, which is controlled of classical heat engines by Sadi Carnot in 1824 has largely by an acousto-optic modulator. By means of video microscopy, the shaped our understanding of work and heat exchange during two-dimensional trajectory was sampled with spatial and temporal macroscopic thermodynamic processes1. Equipped with our resolutions of 10 nm and 33 ms, respectively. Keeping hot and cold present-day ability to design and control mechanical devices at reservoirs thermally isolated at small length scales is experimentally micro- and nanometre length scales, we are now in a position very difficult to achieve, so rather than coupling our colloidal to explore the limitations of classical thermodynamics, arising particle periodically to different heat baths, here we suddenly on scales for which thermal fluctuations are important2–5. Here changed the temperature of the surrounding liquid. we demonstrate the experimental realization of a microscopic This variation of the bath temperature was achieved by a second heat engine, comprising a single colloidal particle subject to a coaxially aligned laser beam whose wavelength was matched to an time-dependent optical laser trap. The work associated with absorption peak of water. This allowed us to heat the suspension the system is a fluctuating quantity, and depends strongly from room temperature to 90 ◦C in less than 10 ms (ref. -

Radiation Exchange Between Surfaces
Chapter 1 Radiation Exchange Between Surfaces 1.1 Motivation and Objectives Thermal radiation, as you know, constitutes one of the three basic modes (or mechanisms) of heat transfer, i.e., conduction, convection, and radiation. Actually, on a physical basis, there are only two mechanisms of heat transfer; diffusion (the transfer of heat via molecular interactions) and radiation (the transfer of heat via photons/electomagnetic waves). Convection, being the bulk transport of a fluid, is not precisely a heat transfer mechanism. The physics of radiation transport are distinctly different than diffusion transport. The latter is a local phenomena, meaning that the rate of diffusion heat transfer, at a point in space, precisely depends only on the local nature about the point, i.e., the temperature gradient and thermal conductivity at the point. Of course, the temperature field will depend on the boundary and initial conditions imposed on the system. However, the diffusion heat flux at, say, one point in the system does not directly effect the diffusion flux at some distant point. Radiation, on the other hand, is not local; the flux of radiation at a point will, in general, be directly and instantaneously dependent on the radiation flux at all points in a system. Unlike diffusion, radiation can act over a distance. Accordingly, the mathematical description of radiation transport will employ an integral equation for the radiation field, as opposed to the differential equation for heat diffusion. Our objectives in studying radiation in the short amount of time left in the course will be to 1. Develop a basic physical understanding of electromagnetic radiation, with emphasis on the properties of radiation that are relevant to heat transfer. -
Properties of Electromagnetic Waves Any Electromagnetic Wave Must Satisfy Four Basic Conditions: 1
Chapter 34 Electromagnetic Waves The Goal of the Entire Course Maxwell’s Equations: Maxwell’s Equations James Clerk Maxwell •1831 – 1879 •Scottish theoretical physicist •Developed the electromagnetic theory of light •His successful interpretation of the electromagnetic field resulted in the field equations that bear his name. •Also developed and explained – Kinetic theory of gases – Nature of Saturn’s rings – Color vision Start at 12:50 https://www.learner.org/vod/vod_window.html?pid=604 Correcting Ampere’s Law Two surfaces S1 and S2 near the plate of a capacitor are bounded by the same path P. Ampere’s Law states that But it is zero on S2 since there is no conduction current through it. This is a contradiction. Maxwell fixed it by introducing the displacement current: Fig. 34-1, p. 984 Maxwell hypothesized that a changing electric field creates an induced magnetic field. Induced Fields . An increasing solenoid current causes an increasing magnetic field, which induces a circular electric field. An increasing capacitor charge causes an increasing electric field, which induces a circular magnetic field. Slide 34-50 Displacement Current d d(EA)d(q / ε) 1 dq E 0 dt dt dt ε0 dt dq d ε E dt0 dt The displacement current is equal to the conduction current!!! Bsd μ I μ ε I o o o d Maxwell’s Equations The First Unified Field Theory In his unified theory of electromagnetism, Maxwell showed that electromagnetic waves are a natural consequence of the fundamental laws expressed in these four equations: q EABAdd 0 εo dd Edd s BE B s μ I μ ε dto o o dt QuickCheck 34.4 The electric field is increasing. -

Hybrid Electrochemical Capacitors
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 1-11-2018 Hybrid Electrochemical Capacitors: Materials, Optimization, and Miniaturization Richa Agrawal Department of Mechanical and Materials Engineering, Florida International University, [email protected] DOI: 10.25148/etd.FIDC006581 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Materials Chemistry Commons, Materials Science and Engineering Commons, Nanoscience and Nanotechnology Commons, and the Physical Chemistry Commons Recommended Citation Agrawal, Richa, "Hybrid Electrochemical Capacitors: Materials, Optimization, and Miniaturization" (2018). FIU Electronic Theses and Dissertations. 3680. https://digitalcommons.fiu.edu/etd/3680 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida HYBRID ELECTROCHEMICAL CAPACITORS: MATERIALS, OPTIMIZATION, AND MINIATURIZATION A dissertation submitted in partial fulfillment of the requirement for the degree of DOCTOR OF PHILOSOPHY in MATERIALS SCIENCE AND ENGINEERING by Richa Agrawal 2018 To: Dean John L. Volakis College of Engineering and Computing This dissertation entitled Hybrid Electrochemical Capacitors: Materials, Optimization, and Miniaturization, written -

Direct Insolation Models
SERI/TR-33S-344 UC CATEGORY: UC-S9,61,62,63 DIRECT INSOLATION MODELS RICHARD BIRD ROLAND L. HULSTROM JANUARY 1980 PREPARED UNDER TASK No. 3623.01 Solar Energy Research Institute 1536 Cole Boulevard Golden. Colorado 80401 A Division of Midwest Research Institute Prepared for the U.S_ Department of Energy Contract No_ EG-77-C-01-4042 , '. Printed in the United States of America Available from: National Technical Information Service U.S. Departm ent of Commerce 5285 Port Royal Road Springfi e1dt VA 22161 Price: Microfiche $3.00 Printed Copy $ 5.25 NOTICE This report was prepared as an account of work sponsored by the United States Govern ment. Neither the United States nor the United States Department of Energy, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness or usefulness of any information, apparatus, product or process dis closed, or represents that its us e would not infringe privately owned rights. S=�I TR-344 FOREWORD This re port documents wo rk pe rforme d by the SERI Energy Resource Assessment Branch for the Division of Solar Energy Technology of the U.S. De pa rtment of Energy. The re po rt compares several simple direct insolation models with a rigorous solar transmission model and describes an improved , simple, direct insolation model. Roland L. Hulstrom , Branch Chief Energy Resource Assessment , \ Approved for: SOLAR ENERGY RE SEARCH INSTITUTE for Research iii S=�I TR-344 TABLE OF CONTENTS 1.0 Introduction •••••••••••••••••••••••••••••••••••••••••••••••••••••••• 1 2.0 Description of Models •••••••••••• .