Chapter 3 Absorption, Emission, Reflection, And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -

Dozy-Chaos Mechanics for a Broad Audience
challenges Concept Paper Dozy-Chaos Mechanics for a Broad Audience Vladimir V. Egorov Russian Academy of Sciences, FSRC “Crystallography and Photonics”, Photochemistry Center, 7a Novatorov Street, 119421 Moscow, Russia; [email protected] Received: 29 June 2020; Accepted: 28 July 2020; Published: 31 July 2020 Abstract: A new and universal theoretical approach to the dynamics of the transient state in elementary physico-chemical processes, called dozy-chaos mechanics (Egorov, V.V. Heliyon Physics 2019, 5, e02579), is introduced to a wide general readership. Keywords: theoretical physics; quantum mechanics; molecular quantum transitions; singularity; dozy chaos; dozy-chaos mechanics; electron transfer; condensed matter; applications 1. Introduction In a publication of Petrenko and Stein [1], it is demonstrated that “the feasibility of the theory of extended multiphonon electron transitions” [2–4] “for the description of optical spectra of polymethine dyes and J-aggregates using quantum chemistry”. A recent publication by the same authors [5] “demonstrates the feasibility and applicability of the theory of extended multiphonon electron transitions for the description of nonlinear optical properties of polymethine dyes using quantum chemistry and model calculations”. About twenty years have passed since the first publications on the theory of extended multiphonon electron transitions [2–4], however, apart from the author of this theory and this paper, only Petrenko and Stein managed to use their results constructively, despite their promising character [1,5,6], and despite the fact that for a long time, the new theory has been constantly in the field of view of a sufficiently large number of researchers (see, e.g., [7–31]). The low demand in practice for the new, promising theory is associated not only with its complexity (however, we note that this theory is the simplest theory of all its likely future and more complex modifications [6]), but also with an insufficiently clear presentation of its physical foundations in the author’s pioneering works [2–4]. -
Plant Lighting Basics and Applications
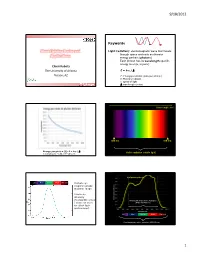
9/18/2012 Keywords Plant Lighting Basics and Light (radiation): electromagnetic wave that travels through space and exits as discrete Applications energy packets (photons) Each photon has its wavelength-specific energy level (E, in joule) Chieri Kubota The University of Arizona E = h·c / Tucson, AZ E: Energy per photon (joule per photon) h: Planck’s constant c: speed of light Greensys 2011, Greece : wavelength (meter) Wavelength (nm) 380 nm 780 nm Energy per photon [J]: E = h·c / Visible radiation (visible light) mole photons = 6.02 x 1023 photons Leaf photosynthesis UV Blue Green Red Far red Human eye response peaks at green range. Luminous intensity (footcandle or lux Photosynthetically Active Radiation ) does not work (PAR, 400-700 nm) for plant light environment. UV Blue Green Red Far red Plant biologically active radiation (300-800 nm) 1 9/18/2012 Chlorophyll a Phytochrome response Chlorophyll b UV Blue Green Red Far red Absorption spectra of isolated chlorophyll Absorptance 400 500 600 700 Wavelength (nm) Daily light integral (DLI or Daily Light Unit and Terminology PPF) Radiation Photons Visible light •Total amount of photosynthetically active “Base” unit Energy (J) Photons (mol) Luminous intensity (cd) radiation (400‐700 nm) received per sq Flux [total amount received Radiant flux Photon flux Luminous flux meter per day or emitted per time] (J s-1) or (W) (mol s-1) (lm) •Unit: mole per sq meter per day (mol m‐2 d‐1) Flux density [total amount Radiant flux density Photon flux (density) Illuminance, • Under optimal conditions, plant growth is received per area per time] (W m-2) (mol m-2 s-1) Luminous flux density highly correlated with DLI. -

12 Light Scattering AQ1
12 Light Scattering AQ1 Lev T. Perelman CONTENTS 12.1 Introduction ......................................................................................................................... 321 12.2 Basic Principles of Light Scattering ....................................................................................323 12.3 Light Scattering Spectroscopy ............................................................................................325 12.4 Early Cancer Detection with Light Scattering Spectroscopy .............................................326 12.5 Confocal Light Absorption and Scattering Spectroscopic Microscopy ............................. 329 12.6 Light Scattering Spectroscopy of Single Nanoparticles ..................................................... 333 12.7 Conclusions ......................................................................................................................... 335 Acknowledgment ........................................................................................................................... 335 References ...................................................................................................................................... 335 12.1 INTRODUCTION Light scattering in biological tissues originates from the tissue inhomogeneities such as cellular organelles, extracellular matrix, blood vessels, etc. This often translates into unique angular, polari- zation, and spectroscopic features of scattered light emerging from tissue and therefore information about tissue -

Solutions 7: Interacting Quantum Field Theory: Λφ4
QFT PS7 Solutions: Interacting Quantum Field Theory: λφ4 (4/1/19) 1 Solutions 7: Interacting Quantum Field Theory: λφ4 Matrix Elements vs. Green Functions. Physical quantities in QFT are derived from matrix elements M which represent probability amplitudes. Since particles are characterised by having a certain three momentum and a mass, and hence a specified energy, we specify such physical calculations in terms of such \on-shell" values i.e. four-momenta where p2 = m2. For instance the notation in momentum space for a ! scattering in scalar Yukawa theory uses four-momenta labels p1 and p2 flowing into the diagram for initial states, with q1 and q2 flowing out of the diagram for the final states, all of which are on-shell. However most manipulations in QFT, and in particular in this problem sheet, work with Green func- tions not matrix elements. Green functions are defined for arbitrary values of four momenta including unphysical off-shell values where p2 6= m2. So much of the information encoded in a Green function has no obvious physical meaning. Of course to extract the corresponding physical matrix element from the Greens function we would have to apply such physical constraints in order to get the physics of scattering of real physical particles. Alternatively our Green function may be part of an analysis of a much bigger diagram so it represents contributions from virtual particles to some more complicated physical process. ∗1. The full propagator in λφ4 theory Consider a theory of a real scalar field φ 1 1 λ L = (@ φ)(@µφ) − m2φ2 − φ4 (1) 2 µ 2 4! (i) A theory with a gφ3=(3!) interaction term will not have an energy which is bounded below. -

TIE-35: Transmittance of Optical Glass
DATE October 2005 . PAGE 1/12 TIE-35: Transmittance of optical glass 0. Introduction Optical glasses are optimized to provide excellent transmittance throughout the total visible range from 400 to 800 nm. Usually the transmittance range spreads also into the near UV and IR regions. As a general trend lowest refractive index glasses show high transmittance far down to short wavelengths in the UV. Going to higher index glasses the UV absorption edge moves closer to the visible range. For highest index glass and larger thickness the absorption edge already reaches into the visible range. This UV-edge shift with increasing refractive index is explained by the general theory of absorbing dielectric media. So it may not be overcome in general. However, due to improved melting technology high refractive index glasses are offered nowadays with better blue-violet transmittance than in the past. And for special applications where best transmittance is required SCHOTT offers improved quality grades like for SF57 the grade SF57 HHT. The aim of this technical information is to give the optical designer a deeper understanding on the transmittance properties of optical glass. 1. Theoretical background A light beam with the intensity I0 falls onto a glass plate having a thickness d (figure 1-1). At the entrance surface part of the beam is reflected. Therefore after that the intensity of the beam is Ii0=I0(1-r) with r being the reflectivity. Inside the glass the light beam is attenuated according to the exponential function. At the exit surface the beam intensity is I i = I i0 exp(-kd) [1.1] where k is the absorption constant. -

Reflectance and Transmittance Sphere
Reflectance and Transmittance Sphere Spectral Evolution four inch portable reflectance/ transmittance sphere is ideal for measuring vegetation physiological states SPECTRAL EVOLUTION offers a portable, compact four inch reflectance/transmittance (R/T) integrating sphere for measuring the reflectance and transmittance of vegetation. The 4 inch R/T sphere is lightweight and portable so you can take it into the field for in situ meas- urements, delivered with a stand as well as a ¼-20 mount for use with tripods. When used with a SPECTRAL EVOLUTION spectrometer or spectroradiometer such as the PSR+, RS-3500, RS- 5400, SR-6500 or RS-8800, it delivers detailed information for measurements of transmittance and reflectance modes. Two varying light intensity levels (High and Low) allow for a broader range of samples to be measured. Light reflected or transmitted from a sample in a sphere is integrated over a full hemisphere, with measurements insensitive to sample anisotropic directional reflectance (transmittance). This provides repeatable measurements of a variety of samples. The 4 inch portable R/T sphere can be used in vegetation studies such as, deriving absorbance characteristics, and estimating the leaf area index (LAI) from radiation reflected from a canopy surface. The sphere allows researchers to limit destructive sampling and provides timely data acquisition of key material samples. The R/T sphere allows you to collect all diffuse light reflected from a sample – measuring total hemispherical reflectance. You can measure reflectance and transmittance, and calculate absorption. You can select the bulb inten- sity level that suits your sample. A white reference is provided for both reflectance and transmittance measurements. -

Applied Spectroscopy Spectroscopic Nomenclature
Applied Spectroscopy Spectroscopic Nomenclature Absorbance, A Negative logarithm to the base 10 of the transmittance: A = –log10(T). (Not used: absorbancy, extinction, or optical density). (See Note 3). Absorptance, α Ratio of the radiant power absorbed by the sample to the incident radiant power; approximately equal to (1 – T). (See Notes 2 and 3). Absorption The absorption of electromagnetic radiation when light is transmitted through a medium; hence ‘‘absorption spectrum’’ or ‘‘absorption band’’. (Not used: ‘‘absorbance mode’’ or ‘‘absorbance band’’ or ‘‘absorbance spectrum’’ unless the ordinate axis of the spectrum is Absorbance.) (See Note 3). Absorption index, k See imaginary refractive index. Absorptivity, α Internal absorbance divided by the product of sample path length, ℓ , and mass concentration, ρ , of the absorbing material. A / α = i ρℓ SI unit: m2 kg–1. Common unit: cm2 g–1; L g–1 cm–1. (Not used: absorbancy index, extinction coefficient, or specific extinction.) Attenuated total reflection, ATR A sampling technique in which the evanescent wave of a beam that has been internally reflected from the internal surface of a material of high refractive index at an angle greater than the critical angle is absorbed by a sample that is held very close to the surface. (See Note 3.) Attenuation The loss of electromagnetic radiation caused by both absorption and scattering. Beer–Lambert law Absorptivity of a substance is constant with respect to changes in path length and concentration of the absorber. Often called Beer’s law when only changes in concentration are of interest. Brewster’s angle, θB The angle of incidence at which the reflection of p-polarized radiation is zero. -

CNT-Based Solar Thermal Coatings: Absorptance Vs. Emittance
coatings Article CNT-Based Solar Thermal Coatings: Absorptance vs. Emittance Yelena Vinetsky, Jyothi Jambu, Daniel Mandler * and Shlomo Magdassi * Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 9190401, Israel; [email protected] (Y.V.); [email protected] (J.J.) * Correspondence: [email protected] (D.M.); [email protected] (S.M.) Received: 15 October 2020; Accepted: 13 November 2020; Published: 17 November 2020 Abstract: A novel approach for fabricating selective absorbing coatings based on carbon nanotubes (CNTs) for mid-temperature solar–thermal application is presented. The developed formulations are dispersions of CNTs in water or solvents. Being coated on stainless steel (SS) by spraying, these formulations provide good characteristics of solar absorptance. The effect of CNT concentration and the type of the binder and its ratios to the CNT were investigated. Coatings based on water dispersions give higher adsorption, but solvent-based coatings enable achieving lower emittance. Interestingly, the binder was found to be responsible for the high emittance, yet, it is essential for obtaining good adhesion to the SS substrate. The best performance of the coatings requires adjusting the concentration of the CNTs and their ratio to the binder to obtain the highest absorptance with excellent adhesion; high absorptance is obtained at high CNT concentration, while good adhesion requires a minimum ratio between the binder/CNT; however, increasing the binder concentration increases the emissivity. The best coatings have an absorptance of ca. 90% with an emittance of ca. 0.3 and excellent adhesion to stainless steel. Keywords: carbon nanotubes (CNTs); binder; dispersion; solar thermal coating; absorptance; emittance; adhesion; selectivity 1. -

Near-Infrared Spectroscopic Analysis for Classification
CORE Metadata, citation and similar papers at core.ac.uk Provided by Wood and Fiber Science (E-Journal) NEAR-INFRARED SPECTROSCOPIC ANALYSIS FOR CLASSIFICATION OF WATER MOLECULES IN WOOD BY A THEORY OF WATER MIXTURES Sang-Yun Yang Graduate Research Assistant Department of Forest Sciences Seoul National University Seoul, Korea 151-921 E-mail: [email protected] Chang-Deuk Eom Research Scientist Korea Forest Research Institute Seoul, Korea E-mail: [email protected] Yeonjung Han PhD Candidate E-mail: [email protected] Yoonseong Chang Graduate Research Assistant E-mail: [email protected] Yonggun Park Department of Forest Sciences Seoul National University Seoul, Korea 151-921 E-mail: [email protected] Jun-Jae Lee Professor E-mail: [email protected] Joon-Weon Choi Associate Professor E-mail: [email protected] Hwanmyeong Yeo*{ Associate Professor Research Institute for Agriculture and Life Sciences Department of Forest Sciences Seoul National University Seoul, Korea, 151-921 E-mail: [email protected] (Received May 2013) * Corresponding author { SWST member Wood and Fiber Science, 46(2), 2014, pp. 138-147 # 2014 by the Society of Wood Science and Technology Yang et al—NIR SPECTROSCOPIC ANALYSIS FOR WOOD WATER MOLECULES 139 Abstract. This study was conducted to analyze the mechanism of moisture adsorption–desorption in wood using near-IR (NIR) spectroscopy. NIR spectra reflected from moist wood were acquired, and spectra in the range from 1800-2100 nm, which were sensitive to water variation, were decomposed into three different components according to the Buijs and Choppin theory. It is assumed that the three components represent three types of bound water: water molecules without -OH groups engaged in hydrogen bonds (S0), water molecules with one -OH group engaged in a hydrogen bond (S1), and water molecules with two -OH groups engaged in hydrogen bonds (S2). -

The Basic Interactions Between Photons and Charged Particles With
Outline Chapter 6 The Basic Interactions between • Photon interactions Photons and Charged Particles – Photoelectric effect – Compton scattering with Matter – Pair productions Radiation Dosimetry I – Coherent scattering • Charged particle interactions – Stopping power and range Text: H.E Johns and J.R. Cunningham, The – Bremsstrahlung interaction th physics of radiology, 4 ed. – Bragg peak http://www.utoledo.edu/med/depts/radther Photon interactions Photoelectric effect • Collision between a photon and an • With energy deposition atom results in ejection of a bound – Photoelectric effect electron – Compton scattering • The photon disappears and is replaced by an electron ejected from the atom • No energy deposition in classical Thomson treatment with kinetic energy KE = hν − Eb – Pair production (above the threshold of 1.02 MeV) • Highest probability if the photon – Photo-nuclear interactions for higher energies energy is just above the binding energy (above 10 MeV) of the electron (absorption edge) • Additional energy may be deposited • Without energy deposition locally by Auger electrons and/or – Coherent scattering Photoelectric mass attenuation coefficients fluorescence photons of lead and soft tissue as a function of photon energy. K and L-absorption edges are shown for lead Thomson scattering Photoelectric effect (classical treatment) • Electron tends to be ejected • Elastic scattering of photon (EM wave) on free electron o at 90 for low energy • Electron is accelerated by EM wave and radiates a wave photons, and approaching • No -

Direct Insolation Models
SERI/TR-33S-344 UC CATEGORY: UC-S9,61,62,63 DIRECT INSOLATION MODELS RICHARD BIRD ROLAND L. HULSTROM JANUARY 1980 PREPARED UNDER TASK No. 3623.01 Solar Energy Research Institute 1536 Cole Boulevard Golden. Colorado 80401 A Division of Midwest Research Institute Prepared for the U.S_ Department of Energy Contract No_ EG-77-C-01-4042 , '. Printed in the United States of America Available from: National Technical Information Service U.S. Departm ent of Commerce 5285 Port Royal Road Springfi e1dt VA 22161 Price: Microfiche $3.00 Printed Copy $ 5.25 NOTICE This report was prepared as an account of work sponsored by the United States Govern ment. Neither the United States nor the United States Department of Energy, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness or usefulness of any information, apparatus, product or process dis closed, or represents that its us e would not infringe privately owned rights. S=�I TR-344 FOREWORD This re port documents wo rk pe rforme d by the SERI Energy Resource Assessment Branch for the Division of Solar Energy Technology of the U.S. De pa rtment of Energy. The re po rt compares several simple direct insolation models with a rigorous solar transmission model and describes an improved , simple, direct insolation model. Roland L. Hulstrom , Branch Chief Energy Resource Assessment , \ Approved for: SOLAR ENERGY RE SEARCH INSTITUTE for Research iii S=�I TR-344 TABLE OF CONTENTS 1.0 Introduction •••••••••••••••••••••••••••••••••••••••••••••••••••••••• 1 2.0 Description of Models •••••••••••• .