2019 Redefinition of SI Base Units
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Learner's Guide to SI Units and Their Conversion
A Learner's Guide to SI Units and their Conversion October 2004 Workbook for students Learner's Guide to SI Units and their Conversion their and Units SI to Guide Learner's Edexcel A London Qualifications is one of the leading examining and awarding bodies in the UK and throughout the world. It incorporates all the qualifications previously awarded under the Edexcel and BTEC brand. We provide a wide range of qualifications including general (academic), vocational, occupational and specific programmes for employers. Through a network of UK and overseas offices, our centres receive the support they need to help them deliver their education and training programmes to learners. For further information please call Customer Services on 0870 240 9800, or visit our website at www.edexcel.org.uk Authorised by Jim Dobson Prepared by Sarah Harrison All the material in this publication is copyright © London Qualifications Limited 2004 CONTENTS Introduction 1 What are units? 2 Operations with units 5 Submultiple and multiple units 9 Conversion of units 11 Conversion examples and exercises 13 Length 13 Area 14 Volume 15 Mass 16 Time 17 Temperature 18 Density 19 Force 20 Stress and pressure 21 Answers to exercises 23 Introduction One of the important areas where Science and Technology students need support is in the conversion of units. This booklet is designed to be useful for students in all Science, Technology and Engineering subjects. This booklet has been produced to: S introduce students to SI base and derived units and S help students with the conversion of multiple and sub-multiple units to SI base and derived units. -

The Avogadro Constant to Be Equal to Exactly 6.02214X×10 23 When It Is Expressed in the Unit Mol −1
[august, 2011] redefinition of the mole and the new system of units zoltan mester It is as easy to count atomies as to resolve the propositions of a lover.. As You Like It William Shakespeare 1564-1616 Argentina, Austria-Hungary, Belgium, Brazil, Denmark, France, German Empire, Italy, Peru, Portugal, Russia, Spain, Sweden and Norway, Switzerland, Ottoman Empire, United States and Venezuela 1875 -May 20 1875, BIPM, CGPM and the CIPM was established, and a three- dimensional mechanical unit system was setup with the base units metre, kilogram, and second. -1901 Giorgi showed that it is possible to combine the mechanical units of this metre–kilogram–second system with the practical electric units to form a single coherent four-dimensional system -In 1921 Consultative Committee for Electricity (CCE, now CCEM) -by the 7th CGPM in 1927. The CCE to proposed, in 1939, the adoption of a four-dimensional system based on the metre, kilogram, second, and ampere, the MKSA system, a proposal approved by the ClPM in 1946. -In 1954, the 10th CGPM, the introduction of the ampere, the kelvin and the candela as base units -in 1960, 11th CGPM gave the name International System of Units, with the abbreviation SI. -in 1970, the 14 th CGMP introduced mole as a unit of amount of substance to the SI 1960 Dalton publishes first set of atomic weights and symbols in 1805 . John Dalton(1766-1844) Dalton publishes first set of atomic weights and symbols in 1805 . John Dalton(1766-1844) Much improved atomic weight estimates, oxygen = 100 . Jöns Jacob Berzelius (1779–1848) Further improved atomic weight estimates . -

Page 1 of 29 Dalton Transactions
Dalton Transactions Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/dalton Page 1 of 29 Dalton Transactions Core-level photoemission spectra of Mo 0.3 Cu 0.7 Sr 2ErCu 2Oy, a superconducting perovskite derivative. Unconventional structure/property relations a, b b b a Sourav Marik , Christine Labrugere , O.Toulemonde , Emilio Morán , M. A. Alario- Manuscript Franco a,* aDpto. Química Inorgánica, Facultad de CC.Químicas, Universidad Complutense de Madrid, 28040- Madrid (Spain) bCNRS, Université de Bordeaux, ICMCB, 87 avenue du Dr. A. Schweitzer, Pessac, F-33608, France -

2.1 Definition of the SI
CCPR/16-53 Modifications to the Draft of the ninth SI Brochure dated 16 September 2016 recommended by the CCPR to the CCU via the CCPR president Takashi Usuda, Wednesday 14 December 2016. The text in black is a selection of paragraphs from the brochure with the section title for indication. The sentences to be modified appear in red. 2.1 Definition of the SI Like for any value of a quantity, the value of a fundamental constant can be expressed as the product of a number and a unit as Q = {Q} [Q]. The definitions below specify the exact numerical value of each constant when its value is expressed in the corresponding SI unit. By fixing the exact numerical value the unit becomes defined, since the product of the numerical value {Q} and the unit [Q] has to equal the value Q of the constant, which is postulated to be invariant. The seven constants are chosen in such a way that any unit of the SI can be written either through a defining constant itself or through products or ratios of defining constants. The International System of Units, the SI, is the system of units in which the unperturbed ground state hyperfine splitting frequency of the caesium 133 atom Cs is 9 192 631 770 Hz, the speed of light in vacuum c is 299 792 458 m/s, the Planck constant h is 6.626 070 040 ×1034 J s, the elementary charge e is 1.602 176 620 8 ×1019 C, the Boltzmann constant k is 1.380 648 52 ×1023 J/K, 23 -1 the Avogadro constant NA is 6.022 140 857 ×10 mol , 12 the luminous efficacy of monochromatic radiation of frequency 540 ×10 hertz Kcd is 683 lm/W. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -
![Arxiv:0801.0028V1 [Physics.Atom-Ph] 29 Dec 2007 § ‡ † (People’S China Metrology, Of) of Lic Institute Canada National Council, Zhang, Research Z](https://docslib.b-cdn.net/cover/1910/arxiv-0801-0028v1-physics-atom-ph-29-dec-2007-%C2%A7-people-s-china-metrology-of-of-lic-institute-canada-national-council-zhang-research-z-651910.webp)
Arxiv:0801.0028V1 [Physics.Atom-Ph] 29 Dec 2007 § ‡ † (People’S China Metrology, Of) of Lic Institute Canada National Council, Zhang, Research Z
CODATA Recommended Values of the Fundamental Physical Constants: 2006∗ Peter J. Mohr†, Barry N. Taylor‡, and David B. Newell§, National Institute of Standards and Technology, Gaithersburg, Maryland 20899-8420, USA (Dated: March 29, 2012) This paper gives the 2006 self-consistent set of values of the basic constants and conversion factors of physics and chemistry recommended by the Committee on Data for Science and Technology (CODATA) for international use. Further, it describes in detail the adjustment of the values of the constants, including the selection of the final set of input data based on the results of least-squares analyses. The 2006 adjustment takes into account the data considered in the 2002 adjustment as well as the data that became available between 31 December 2002, the closing date of that adjustment, and 31 December 2006, the closing date of the new adjustment. The new data have led to a significant reduction in the uncertainties of many recommended values. The 2006 set replaces the previously recommended 2002 CODATA set and may also be found on the World Wide Web at physics.nist.gov/constants. Contents 3. Cyclotron resonance measurement of the electron relative atomic mass Ar(e) 8 Glossary 2 4. Atomic transition frequencies 8 1. Introduction 4 1. Hydrogen and deuterium transition frequencies, the 1. Background 4 Rydberg constant R∞, and the proton and deuteron charge radii R , R 8 2. Time variation of the constants 5 p d 1. Theory relevant to the Rydberg constant 9 3. Outline of paper 5 2. Experiments on hydrogen and deuterium 16 3. -

Indoor Selectable- Output Horns, Strobes, and Horn Strobes for Wall Applications
Indoor Selectable- Output Horns, Strobes, and Horn Strobes for Wall Applications SpectrAlert® Advance audible visible notification products are rich with features guaranteed to cut installation times and maximize profits. Features The SpectrAlert Advance series offers the most versatile and easy-to-use line of horns, strobes, and horn strobes in the industry. • Plug-in design with minimal intrusion into the back box With white and red plastic housings, wall and ceiling mounting • Tamper-resistant construction options, and plain and FIRE-printed devices, SpectrAlert Advance • Automatic selection of 12- or 24-volt operation at can meet virtually any application requirement. 15 and 15/75 candela Like the entire SpectrAlert Advance product line, wall-mount horns, • Field-selectable candela settings on wall units: 15, 15/75, strobes, and horn strobes include a variety of features that increase 30, 75, 95, 110, 115, 135, 150, 177, and 185 their application versatility while simplifying installation. All devices • Horn rated at 88+ dBA at 16 volts feature plug-in designs with minimal intrusion into the back box, • Rotary switch for horn tone and three volume selections making installations fast and foolproof while virtually eliminating costly and time-consuming ground faults. • Universal mounting plate for wall units • Mounting plate shorting spring checks wiring continuity before To further simplify installation and protect devices from construction device installation damage, SpectrAlert Advance utilizes a universal mounting plate • Electrically Compatible with legacy SpectrAlert devices with an onboard shorting spring, so installers can test wiring continuity before the device is installed. • Compatible with MDL3 sync module • Listed for ceiling or wall mounting Installers can also easily adapt devices to a suit a wide range of application requirements using field-selectable candela settings, automatic selection of 12- or 24-volt operation, and a rotary switch for horn tones with three volume selections. -

Worksheet 2 the Metric System and SI Units
Unit Conversions Worksheet 2 The Metric System and SI Units 1 © MathTutorDVD.com Here is a helpful list of all the prefixes and their exponents and abbreviations: 109 giga G one billion 106 mega M one million 103 kilo k one thousand 102 hecto h one hundred 101 deca da ten 10-1 deci d one tenth 10-2 centi c one hundredth 10-3 milli m one thousandth 10-6 micro µ one millionth 10-9 nano n one billionth 10-12 pico p one trillionth 2 © MathTutorDVD.com 1. Give the abbreviation for each of the SI base units below. a. kilogram b. Kelvin c. meter d. second 2. State what quantities the following SI units are used to measure. a. kg b. m c. s d. K 3. Give the power of 10 that each of the following prefixes represents. a. kilo b. micro c. centi d. deca e. mega 3 © MathTutorDVD.com 4. Write the following numbers in their base units. a. 5 kilometers b. 12 centimeters c. 2 micrograms d. 4.1 megagrams 5. Write the abbreviations for the following measurements. a. 67 micrograms b. 831 kilometers c. 1.2 meters d. 791 megagrams 4 © MathTutorDVD.com 6. Express the following numbers with an appropriate SI prefix (e.g., 5,000 g = 5 kg). a. 7800 m b. 5.0 x 10-6 g c. 7.8 x 106 m d. 1.6 x 10-3 g 7. Convert the following to scientific notation using the base SI unit. a. 4.51 microseconds b. 6700 grams c. -

The International System of Units (SI)
NAT'L INST. OF STAND & TECH NIST National Institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 330 2001 Edition The International System of Units (SI) 4. Barry N. Taylor, Editor r A o o L57 330 2oOI rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -

Standards and Units: a View from the President of the Royal Society of New South Wales
Journal & Proceedings of the Royal Society of New South Wales, vol. 150, part 2, 2017, pp. 143–151. ISSN 0035-9173/17/020143-09 Standards and units: a view from the President of the Royal Society of New South Wales D. Brynn Hibbert The Royal Society of New South Wales, UNSW Sydney, and The International Union of Pure and Applied Chemistry Email: [email protected] Abstract As the Royal Society of New South Wales continues to grow in numbers and influence, the retiring president reflects on the achievements of the Society in the 21st century and describes the impending changes in the International System of Units. Scientific debates that have far reaching social effects should be the province of an Enlightenment society such as the RSNSW. Introduction solved by science alone. Our own Society t may be a long bow, but the changes in embraces “science literature philosophy and Ithe definitions of units used across the art” and we see with increasing clarity that world that have been decades in the making, our business often spans all these fields. As might have resonances in the resurgence in we shall learn the choice of units with which the fortunes of the RSNSW in the 21st cen- to measure our world is driven by science, tury. First, we have a system of units tracing philosophy, history and a large measure of back to the nineteenth century that starts social acceptability, not to mention the occa- with little traction in the world but eventu- sional forearm of a Pharaoh. ally becomes the bedrock of science, trade, Measurement health, indeed any measurement-based activ- ity. -
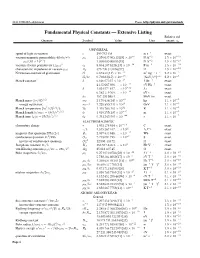
Fundamental Physical Constants — Extensive Listing Relative Std
2018 CODATA adjustment From: http://physics.nist.gov/constants Fundamental Physical Constants — Extensive Listing Relative std. Quantity Symbol Value Unit uncert. ur UNIVERSAL speed of light in vacuum c 299 792 458 m s−1 exact 2 −6 −2 −10 vacuum magnetic permeability 4pα¯h=e c µ0 1:256 637 062 12(19) × 10 NA 1:5 × 10 −7 −2 −10 µ0=(4p × 10 ) 1:000 000 000 55(15) NA 1:5 × 10 2 −12 −1 −10 vacuum electric permittivity 1/µ0c 0 8:854 187 8128(13) × 10 F m 1:5 × 10 −10 characteristic impedance of vacuum µ0c Z0 376:730 313 668(57) Ω 1:5 × 10 Newtonian constant of gravitation G 6:674 30(15) × 10−11 m3 kg−1 s−2 2:2 × 10−5 G=¯hc 6:708 83(15) × 10−39 (GeV=c2)−2 2:2 × 10−5 Planck constant∗ h 6:626 070 15 × 10−34 J Hz−1 exact 4:135 667 696 ::: × 10−15 eV Hz−1 exact ¯h 1:054 571 817 ::: × 10−34 J s exact 6:582 119 569 ::: × 10−16 eV s exact ¯hc 197:326 980 4 ::: MeV fm exact 1=2 −8 −5 Planck mass (¯hc=G) mP 2:176 434(24) × 10 kg 1:1 × 10 2 19 −5 energy equivalent mPc 1:220 890(14) × 10 GeV 1:1 × 10 5 1=2 32 −5 Planck temperature (¯hc =G) =k TP 1:416 784(16) × 10 K 1:1 × 10 3 1=2 −35 −5 Planck length ¯h=mPc = (¯hG=c ) lP 1:616 255(18) × 10 m 1:1 × 10 5 1=2 −44 −5 Planck time lP=c = (¯hG=c ) tP 5:391 247(60) × 10 s 1:1 × 10 ELECTROMAGNETIC elementary charge e 1:602 176 634 × 10−19 C exact e=¯h 1:519 267 447 ::: × 1015 AJ−1 exact −15 magnetic flux quantum 2p¯h=(2e) Φ0 2:067 833 848 ::: × 10 Wb exact 2 −5 conductance quantum 2e =2p¯h G0 7:748 091 729 ::: × 10 S exact −1 inverse of conductance quantum G0 12 906:403 72 ::: Ω exact 9 −1 Josephson constant 2e=h -

Measuring Luminance with a Digital Camera
® Advanced Test Equipment Rentals Established 1981 www.atecorp.com 800-404-ATEC (2832) Measuring Luminance with a Digital Camera Peter D. Hiscocks, P.Eng Syscomp Electronic Design Limited [email protected] www.syscompdesign.com September 16, 2011 Contents 1 Introduction 2 2 Luminance Standard 3 3 Camera Calibration 6 4 Example Measurement: LED Array 9 5 Appendices 11 3.1 LightMeasurementSymbolsandUnits. 11 3.2 TypicalValuesofLuminance.................................... 11 3.3 AccuracyofPhotometricMeasurements . 11 3.4 PerceptionofBrightnessbytheHumanVisionSystem . 12 3.5 ComparingIlluminanceMeters. 13 3.6 FrostedIncandescentLampCalibration . 14 3.7 LuminanceCalibrationusingMoon,SunorDaylight . 17 3.8 ISOSpeedRating.......................................... 17 3.9 WorkFlowSummary ........................................ 18 3.10 ProcessingScripts.......................................... 18 3.11 UsingImageJToDeterminePixelValue . 18 3.12 UsingImageJToGenerateaLuminance-EncodedImage . 19 3.13 EXIFData.............................................. 19 References 22 1 Introduction There is growing awareness of the problem of light pollution, and with that an increasing need to be able to measure the levels and distribution of light. This paper shows how such measurements may be made with a digital camera. Light measurements are generally of two types: illuminance and lumi- nance. Illuminance is a measure of the light falling on a surface, measured in lux. Illuminanceis widely used by lighting designers to specify light levels. In the assessment of light pollution, horizontal and vertical measurements of illuminance are used to assess light trespass and over lighting. Luminance is the measure of light radiating from a source, measured in candela per square meter. Luminance is perceived by the human viewer as the brightness of a light source. In the assessment of light pollution, (a) Lux meter luminance can be used to assess glare, up-light and spill-light1.