American and BRITISH UNITS of Measurement to SI UNITS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix A: Symbols and Prefixes
Appendix A: Symbols and Prefixes (Appendix A last revised November 2020) This appendix of the Author's Kit provides recommendations on prefixes, unit symbols and abbreviations, and factors for conversion into units of the International System. Prefixes Recommended prefixes indicating decimal multiples or submultiples of units and their symbols are as follows: Multiple Prefix Abbreviation 1024 yotta Y 1021 zetta Z 1018 exa E 1015 peta P 1012 tera T 109 giga G 106 mega M 103 kilo k 102 hecto h 10 deka da 10-1 deci d 10-2 centi c 10-3 milli m 10-6 micro μ 10-9 nano n 10-12 pico p 10-15 femto f 10-18 atto a 10-21 zepto z 10-24 yocto y Avoid using compound prefixes, such as micromicro for pico and kilomega for giga. The abbreviation of a prefix is considered to be combined with the abbreviation/symbol to which it is directly attached, forming with it a new unit symbol, which can be raised to a positive or negative power and which can be combined with other unit abbreviations/symbols to form abbreviations/symbols for compound units. For example: 1 cm3 = (10-2 m)3 = 10-6 m3 1 μs-1 = (10-6 s)-1 = 106 s-1 1 mm2/s = (10-3 m)2/s = 10-6 m2/s Abbreviations and Symbols Whenever possible, avoid using abbreviations and symbols in paragraph text; however, when it is deemed necessary to use such, define all but the most common at first use. The following is a recommended list of abbreviations/symbols for some important units. -

Pressure, Its Units of Measure and Pressure References
_______________ White Paper Pressure, Its Units of Measure and Pressure References Viatran Phone: 1‐716‐629‐3800 3829 Forest Parkway Fax: 1‐716‐693‐9162 Suite 500 [email protected] Wheatfield, NY 14120 www.viatran.com This technical note is a summary reference on the nature of pressure, some common units of measure and pressure references. Read this and you won’t have to wait for the movie! PRESSURE Gas and liquid molecules are in constant, random motion called “Brownian” motion. The average speed of these molecules increases with increasing temperature. When a gas or liquid molecule collides with a surface, momentum is imparted into the surface. If the molecule is heavy or moving fast, more momentum is imparted. All of the collisions that occur over a given area combine to result in a force. The force per unit area defines the pressure of the gas or liquid. If we add more gas or liquid to a constant volume, then the number of collisions must increase, and therefore pressure must increase. If the gas inside the chamber is heated, the gas molecules will speed up, impact with more momentum and pressure increases. Pressure and temperature therefore are related (see table at right). The lowest pressure possible in nature occurs when there are no molecules at all. At this point, no collisions exist. This condition is known as a pure vacuum, or the absence of all matter. It is also possible to cool a liquid or gas until all molecular motion ceases. This extremely cold temperature is called “absolute zero”, which is -459.4° F. -

Standard Conversion Factors
Standard conversion factors 7 1 tonne of oil equivalent (toe) = 10 kilocalories = 396.83 therms = 41.868 GJ = 11,630 kWh 1 therm = 100,000 British thermal units (Btu) The following prefixes are used for multiples of joules, watts and watt hours: kilo (k) = 1,000 or 103 mega (M) = 1,000,000 or 106 giga (G) = 1,000,000,000 or 109 tera (T) = 1,000,000,000,000 or 1012 peta (P) = 1,000,000,000,000,000 or 1015 WEIGHT 1 kilogramme (kg) = 2.2046 pounds (lb) VOLUME 1 cubic metre (cu m) = 35.31 cu ft 1 pound (lb) = 0.4536 kg 1 cubic foot (cu ft) = 0.02832 cu m 1 tonne (t) = 1,000 kg 1 litre = 0.22 Imperial = 0.9842 long ton gallon (UK gal.) = 1.102 short ton (sh tn) 1 UK gallon = 8 UK pints 1 Statute or long ton = 2,240 lb = 1.201 U.S. gallons (US gal) = 1.016 t = 4.54609 litres = 1.120 sh tn 1 barrel = 159.0 litres = 34.97 UK gal = 42 US gal LENGTH 1 mile = 1.6093 kilometres 1 kilometre (km) = 0.62137 miles TEMPERATURE 1 scale degree Celsius (C) = 1.8 scale degrees Fahrenheit (F) For conversion of temperatures: °C = 5/9 (°F - 32); °F = 9/5 °C + 32 Average conversion factors for petroleum Imperial Litres Imperial Litres gallons per gallons per per tonne tonne per tonne tonne Crude oil: Gas/diesel oil: Indigenous Gas oil 257 1,167 Imported Marine diesel oil 253 1,150 Average of refining throughput Fuel oil: Ethane All grades 222 1,021 Propane Light fuel oil: Butane 1% or less sulphur 1,071 Naphtha (l.d.f.) >1% sulphur 232 1,071 Medium fuel oil: Aviation gasoline 1% or less sulphur 237 1,079 >1% sulphur 1,028 Motor -
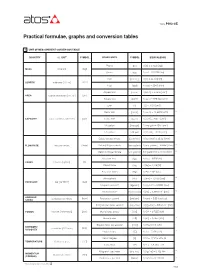
Practical Formulae, Graphs and Conversion Tables
Table P003-4/E Practical formulae, graphs and conversion tables 1 UNIT OF MEASUREMENT CONVERSION TABLE QUANTITY S.I. UNIT SYMBOL OTHER UNITS SYMBOL EQUIVALENCE Pound [lb] 1 [lb] = 0,4536 [kg] kilogram [kg] MASS Ounce [oz] 1 [oz] = 0,02335 [kg] Inch [in] or [”] 1 [in] = 25,40 [mm] millimeter [10-3 m] [mm] LENGTH Foot [foot] 1 [foot] = 304,8 [mm] Square inch [sq in] 1 [sq in] = 6,4516 [cm2] -4 2 [cm2] AREA square centimeter [10 m ] Square foot [sq ft] 1 [sq ft] = 929,034 [cm2] Liter [l] 1 [l] = 1000 [cm3] Cubic inch [cu in] 1 [cu in] = 16,3870 [cm3] * cubic centimeter [10-6 m3] [cm3] Cubic foot [cu ft] 1 [cu ft] = 28317 [cm3] CAPACITY UK gallon [Imp gal] 1 [Imp gal] = 4546 [cm3] US gallon [US gal] 1 [US gal] = 3785 [cm3] * Cubic foot per minute [cu ft/min] 1 [cu ft/min] = 28,32 [l/min] liter per minute [l/min] Gallon (UK) per minute [Imp gal/min] 1 [Imp gal/min] = 4,5456 [l/min] * FLOW RATE Gallon (US) per minute [US gal/min] [US gal/min] = 3,7848 [l/min] * Kilogram force [kgf] 1 [kgf] = 9,806 [N] Newton [kgm/s2] [N] FORCE Pound force [lbf] 1 [lbf] = 4,448 [N] Pascal [1 N/m2] [Pa] 1 [Pa] = 10-5 [bar] Atmosphere [atm] 1 [atm] = 1,0132 [bar] * bar [105 N/m2] [bar] PRESSURE 2 2 2 Kilogram force/cm [kgf/cm ] 1 [kgf/cm ] = 0,9806 [bar] 2 2 -2 Pound force/in [lbf /in ] or [psi] 1 [psi] = 6,8948•10 [bar] * ANGULAR revolution per minute [rpm] Radian per second [rad/sec] 1 [rpm] = 9,55 [rad/sec] SPEED -3 Kilogram per meter second [kgf •m/s] 1 [kgf •m/s] = 9,803•10 [kW] kilowatt [1000 Nm/s] [kW] Metric horse power [CV] 1 [CV] = 0,7355 [kW] POWER -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Pressure Measurement Explained
Pressure measurement explained Rev A1, May 25th, 2018 Sens4Knowledge Sens4 A/S – Nordre Strandvej 119 G – 3150 Hellebaek – Denmark Phone: +45 8844 7044 – Email: [email protected] www.sens4.com Sens4Knowledge Pressure measurement explained Introduction Pressure is defined as the force per area that can be exerted by a liquid, gas or vapor etc. on a given surface. The applied pressure can be measured as absolute, gauge or differential pressure. Pressure can be measured directly by measurement of the applied force or indirectly, e.g. by the measurement of the gas properties. Examples of indirect measurement techniques that are using gas properties are thermal conductivity or ionization of gas molecules. Before mechanical manometers and electronic diaphragm pressure sensors were invented, pressure was measured by liquid manometers with mercury or water. Pressure standards In physical science the symbol for pressure is p and the SI (abbreviation from French Le Système. International d'Unités) unit for measuring pressure is pascal (symbol: Pa). One pascal is the force of one Newton per square meter acting perpendicular on a surface. Other commonly used pressure units for stating the pressure level are psi (pounds per square inch), torr and bar. Use of pressure units have regional and applicational preference: psi is commonly used in the United States, while bar the preferred unit of measure in Europe. In the industrial vacuum community, the preferred pressure unit is torr in the United States, mbar in Europe and pascal in Asia. Unit conversion Pa bar psi torr atm 1 Pa = 1 1×10-5 1.45038×10-4 7.50062×10-3 9.86923×10-6 1 bar = 100,000 1 14.5038 750.062 0.986923 1 psi = 6,894.76 6.89476×10-2 1 51.7149 6.80460×10-2 1 torr = 133.322 1.33322×10-3 1.933768×10-2 1 1.31579×10-3 1 atm (standard) = 1013.25 1.01325 14.6959 760.000 1 According to the International Organization for Standardization the standard ISO 2533:1975 defines the standard atmospheric pressure of 101,325 Pa (1 atm, 1013.25 mbar or 14.6959 psi). -

Common Units
Civil Engineering Hydraulics Pressure and Fluid Statics Leonard: It wouldn't kill us to meet new people. Sheldon: For the record, it could kill us to meet new people. Common Units ¢ In order to be able to discuss and analyze fluid problems we need to be able to understand some fundamental terms commonly used 2 Pressure 1 Common Units ¢ The most used term in hydraulics and fluid mechanics is probably pressure ¢ Pressure is defined as the normal force exerted by a fluid per unit of area l The important part of that definition is the normal (perpendicular) to the unit of area 3 Pressure Common Units ¢ The Pascal is a very small unit of pressure so it is most often encountered with a prefix to allow the numerical values to be easy to display ¢ Common prefixes are the Kilopascal (kPa=103Pa), the Megapascal (MPa=106Pa), and sometimes the Gigapascal (GPa=109Pa) 4 Pressure 2 Common Units ¢ A bar is defined as 105 Pa so a millibar (mbar) is defined as 10-3 bar so the millibar is 102 Pa The word bar finds its origin in the Greek word báros, meaning weight. 5 Pressure Common Units ¢ Standard atmospheric pressure or "the standard atmosphere" (1 atm) is defined as 101.325 kilopascals (kPa). 6 Pressure 3 Common Units ¢ This "standard pressure" is a purely arbitrary representative value for pressure at sea level, and real atmospheric pressures vary from place to place and moment to moment everywhere in the world. 7 Pressure Common Units ¢ Pressure is usually given in reference to some datum l Absolute pressure is given in reference to a system with -

Units of Weight and Measure : Definitions and Tables of Equivalents
Units of Weight and Measure (United States Customary and Metric) Definitions and Tables of Equivalents United States Department of Commerce National Bureau of Standards Miscellaneous Publication 233 THE NATIONAL BUREAU OF STANDARDS Functions and Activities The functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended by Congress in Public Law 619, 1950. These include the development and maintenance of the national standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials, devices, and structures; advisory services to government agencies on scientific and technical problems; invention and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, development, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. Research projects are also performed for other government agencies when the work relates to and supplements the basic program of the Bureau or when the Bureau's unique competence is required. The scope of activities is suggested by the fisting of divisions and sections on the inside of the back cover. Publications The results of the Bureau's work take the form of either actual equipment and devices -

Imperial Units
Imperial units From Wikipedia, the free encyclopedia Jump to: navigation, search This article is about the post-1824 measures used in the British Empire and countries in the British sphere of influence. For the units used in England before 1824, see English units. For the system of weight, see Avoirdupois. For United States customary units, see Customary units . Imperial units or the imperial system is a system of units, first defined in the British Weights and Measures Act of 1824, later refined (until 1959) and reduced. The system came into official use across the British Empire. By the late 20th century most nations of the former empire had officially adopted the metric system as their main system of measurement. The former Weights and Measures office in Seven Sisters, London. Contents [hide] • 1 Relation to other systems • 2 Units ○ 2.1 Length ○ 2.2 Area ○ 2.3 Volume 2.3.1 British apothecaries ' volume measures ○ 2.4 Mass • 3 Current use of imperial units ○ 3.1 United Kingdom ○ 3.2 Canada ○ 3.3 Australia ○ 3.4 Republic of Ireland ○ 3.5 Other countries • 4 See also • 5 References • 6 External links [edit] Relation to other systems The imperial system is one of many systems of English or foot-pound-second units, so named because of the base units of length, mass and time. Although most of the units are defined in more than one system, some subsidiary units were used to a much greater extent, or for different purposes, in one area rather than the other. The distinctions between these systems are often not drawn precisely. -

English Customary Weights and Measures
English Customary Weights and Measures Distance In all traditional measuring systems, short distance units are based on the dimensions of the human body. The inch represents the width of a thumb; in fact, in many languages, the word for "inch" is also the word for "thumb." The foot (12 inches) was originally the length of a human foot, although it has evolved to be longer than most people's feet. The yard (3 feet) seems to have gotten its start in England as the name of a 3-foot measuring stick, but it is also understood to be the distance from the tip of the nose to the end of the middle finger of the outstretched hand. Finally, if you stretch your arms out to the sides as far as possible, your total "arm span," from one fingertip to the other, is a fathom (6 feet). Historically, there are many other "natural units" of the same kind, including the digit (the width of a finger, 0.75 inch), the nail (length of the last two joints of the middle finger, 3 digits or 2.25 inches), the palm (width of the palm, 3 inches), the hand (4 inches), the shaftment (width of the hand and outstretched thumb, 2 palms or 6 inches), the span (width of the outstretched hand, from the tip of the thumb to the tip of the little finger, 3 palms or 9 inches), and the cubit (length of the forearm, 18 inches). In Anglo-Saxon England (before the Norman conquest of 1066), short distances seem to have been measured in several ways. -

Appendix C. General Tables of Units of Measurement
Handbook 44 – 2016 Appendix C – General Tables of Units of Measurement Table of Contents Appendix C. General Tables of Units of Measurement ........................................................ C-3 1. Tables of Metric Units of Measurement ..................................................................................................... C-3 Units of Length ............................................................................................................................................... C-3 Units of Area .................................................................................................................................................. C-3 Units of Liquid Volume .................................................................................................................................. C-4 Units of Volume ............................................................................................................................................. C-4 Units of Mass .................................................................................................................................................. C-4 2. Tables of U.S. Customary Units of Measurement ..................................................................................... C-4 Units of Length ............................................................................................................................................... C-4 Units of Area ................................................................................................................................................. -

Dimensions and Units English Units of Measurement Units of Weight
English units of measurement Today: A system of weights and measures used in a few nations, the only major industrial one Chapter 6 continued: being the United States. It actually consists of Dimensions and Units two related systems—the U.S. Customary System of units, used in the United States and dependencies, and the British Imperial System. Units of Weight The pound (lb) is the basic unit of weight (which is proportional to mass) (how?). Within the English units of measurement there are three different systems of weights. In the avoirdupois system, the most widely used of Question: the three, the pound is divided into 16 ounces (oz) and the ounce into 16 drams. The ton, used Is there a problem? to measure large masses, is equal to 2,000 lb (short ton) or 2,240 lb (long ton). In Great Britain the stone, equal to 14 lb, is also used. “weight is proportional to mass.” Answer: You need to be aware of Force = Mass* Acceleration the law governing that proportionality: Newton’s Law Acceleration is NOT a constant, mass is. Force = Mass* Acceleration Even on earth, g = 9.81 m/s2 is NOT constant, but varies with latitude and Question: elevation. Is there a problem? “weight is proportional to mass.” “weight is proportional to mass.” 1 Another problem arises from the A mass is NEVER a “weight”. common intermingling of the terms “mass” and “weight”, as in: Force = Mass* Acceleration “How much does a pound of mass weigh?” Or: “If you don’t know whether it’s pound- “Weight” = Force mass or pound-weight, simply say pounds.” “Weight” = Force Forces in SI Units …because on earth all masses are 2 exposed to gravity.