Quick Reference Guide on the Units of Measure in Hyperbaric Medicine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

“Body Temperature & Pressure, Saturated” & Ambient Pressure Correction in Air Medical Transport
“Body Temperature & Pressure, Saturated” & Ambient Pressure Correction in air medical transport Mechanical ventilation can be especially challenging during air medical transport, particularly due to the impact of varying atmospheric pressure with changing altitudes. The Oxylog® 3000 plus and Oxylog® 2000 plus help to effectively deal with these challenges. D-33481-2011 Artificial ventilation uses compressed the ventilation volumes delivered by the gas to deliver the required volume to the ventilator. Mechanical ventilation in fixed patient. This breathing gas has normally wing aircraft without a pressurized cabin an ambient temperature level and is very is subject to the same dynamics. In case dry. Inside the human lungs the gas of a pressurized cabin it is still relevant expands due to a higher temperature and to correct the inspiratory volumes, as humidity level. These physical conditions the cabin is usually maintained at MT-5809-2008 are described as “Body Temperature & a pressure of approximately 800 mbar Figure 1: Oxylog® 3000 plus Pressure, Saturated” (BTPS), which (600 mmHg), comparable to an altitude The Oxylog® 3000 plus automatically com- presumes the combined environmental of 8,200 ft/2,500 m. pensates volume delivery and measurement circumstances of – Without BTPS correction, the deli- – a body temperature of 37 °C / 99 °F vered inspiratory volume can deviate up – ambient barometrical pressure to 14 % conditions and (at 14,800 ft/4,500 m altitude) from – breathing gas saturated with water the targeted set volume (i.e. 570 ml vapour (= 100 % relative humidity). instead of 500 ml). – Without ambient pressure correction, Aside from the challenge of changing the inspiratory volume can deviate up temperatures and humidity inside the to 44 % (at 14,800 ft/4,500 m altitude) patient lungs, the ambient pressure is from the targeted set volume also important to consider. -

Pressure, Its Units of Measure and Pressure References
_______________ White Paper Pressure, Its Units of Measure and Pressure References Viatran Phone: 1‐716‐629‐3800 3829 Forest Parkway Fax: 1‐716‐693‐9162 Suite 500 [email protected] Wheatfield, NY 14120 www.viatran.com This technical note is a summary reference on the nature of pressure, some common units of measure and pressure references. Read this and you won’t have to wait for the movie! PRESSURE Gas and liquid molecules are in constant, random motion called “Brownian” motion. The average speed of these molecules increases with increasing temperature. When a gas or liquid molecule collides with a surface, momentum is imparted into the surface. If the molecule is heavy or moving fast, more momentum is imparted. All of the collisions that occur over a given area combine to result in a force. The force per unit area defines the pressure of the gas or liquid. If we add more gas or liquid to a constant volume, then the number of collisions must increase, and therefore pressure must increase. If the gas inside the chamber is heated, the gas molecules will speed up, impact with more momentum and pressure increases. Pressure and temperature therefore are related (see table at right). The lowest pressure possible in nature occurs when there are no molecules at all. At this point, no collisions exist. This condition is known as a pure vacuum, or the absence of all matter. It is also possible to cool a liquid or gas until all molecular motion ceases. This extremely cold temperature is called “absolute zero”, which is -459.4° F. -
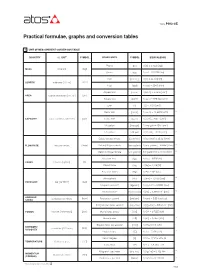
Practical Formulae, Graphs and Conversion Tables
Table P003-4/E Practical formulae, graphs and conversion tables 1 UNIT OF MEASUREMENT CONVERSION TABLE QUANTITY S.I. UNIT SYMBOL OTHER UNITS SYMBOL EQUIVALENCE Pound [lb] 1 [lb] = 0,4536 [kg] kilogram [kg] MASS Ounce [oz] 1 [oz] = 0,02335 [kg] Inch [in] or [”] 1 [in] = 25,40 [mm] millimeter [10-3 m] [mm] LENGTH Foot [foot] 1 [foot] = 304,8 [mm] Square inch [sq in] 1 [sq in] = 6,4516 [cm2] -4 2 [cm2] AREA square centimeter [10 m ] Square foot [sq ft] 1 [sq ft] = 929,034 [cm2] Liter [l] 1 [l] = 1000 [cm3] Cubic inch [cu in] 1 [cu in] = 16,3870 [cm3] * cubic centimeter [10-6 m3] [cm3] Cubic foot [cu ft] 1 [cu ft] = 28317 [cm3] CAPACITY UK gallon [Imp gal] 1 [Imp gal] = 4546 [cm3] US gallon [US gal] 1 [US gal] = 3785 [cm3] * Cubic foot per minute [cu ft/min] 1 [cu ft/min] = 28,32 [l/min] liter per minute [l/min] Gallon (UK) per minute [Imp gal/min] 1 [Imp gal/min] = 4,5456 [l/min] * FLOW RATE Gallon (US) per minute [US gal/min] [US gal/min] = 3,7848 [l/min] * Kilogram force [kgf] 1 [kgf] = 9,806 [N] Newton [kgm/s2] [N] FORCE Pound force [lbf] 1 [lbf] = 4,448 [N] Pascal [1 N/m2] [Pa] 1 [Pa] = 10-5 [bar] Atmosphere [atm] 1 [atm] = 1,0132 [bar] * bar [105 N/m2] [bar] PRESSURE 2 2 2 Kilogram force/cm [kgf/cm ] 1 [kgf/cm ] = 0,9806 [bar] 2 2 -2 Pound force/in [lbf /in ] or [psi] 1 [psi] = 6,8948•10 [bar] * ANGULAR revolution per minute [rpm] Radian per second [rad/sec] 1 [rpm] = 9,55 [rad/sec] SPEED -3 Kilogram per meter second [kgf •m/s] 1 [kgf •m/s] = 9,803•10 [kW] kilowatt [1000 Nm/s] [kW] Metric horse power [CV] 1 [CV] = 0,7355 [kW] POWER -

American and BRITISH UNITS of Measurement to SI UNITS
AMERICAN AND BRITISH UNITS OF MEASUREMENT TO SI UNITS UNIT & ABBREVIATION SI UNITS CONVERSION* UNIT & ABBREVIATION SI UNITS CONVERSION* UNITS OF LENGTH UNITS OF MASS 1 inch = 40 lines in 2.54 cm 0.393701 1 grain gr 64.7989 mg 0.0154324 1 mil 25.4 µm 0.03937 1 dram dr 1.77185 g 0.564383 1 line 0.635 mm 1.57480 1 ounce = 16 drams oz 28.3495 g 0.0352739 1 foot = 12 in = 3 hands ft 30.48 cm 0.0328084 1 pound = 16 oz lb 0.453592 kg 2.204622 1 yard = 3 feet = 4 spans yd 0.9144 m 1.09361 1 quarter = 28 lb 12.7006 kg 0.078737 1 fathom = 2 yd fath 1.8288 m 0.546807 1 hundredweight = 112 lb cwt 50.8024 kg 0.0196841 1 rod (perch, pole) rd 5.0292 m 0.198839 1 long hundredweight l cwt 50.8024 kg 0.0196841 1 chain = 100 links ch 20.1168 m 0.0497097 1 short hundredweight sh cwt 45.3592 kg 0.0220462 1 furlong = 220 yd fur 0.201168 km 4.97097 1 ton = 1 long ton tn, l tn 1.016047 t 0.984206 1 mile (Land Mile) mi 1.60934 km 0.62137 1 short ton = 2000 lb sh tn 0.907185 t 1.102311 1 nautical mile (intl.) n mi, NM 1.852 km 0.539957 1 knot (Knoten) kn 1.852 km/h 0.539957 UNITS OF FORCE 1 pound-weight lb wt 4.448221 N 0.2248089 UNITS OF AREA 1 pound-force LB, lbf 4.448221 N 0.2248089 1 square inch sq in 6.4516 cm2 0.155000 1 poundal pdl 0.138255 N 7.23301 1 circular inch 5.0671 cm2 0.197352 1 kilogram-force kgf, kgp 9.80665 N 0.1019716 1 square foot = 144 sq in sq ft 929.03 cm2 1.0764 x 10-4 1 short ton-weight sh tn wt 8.896444 kN 0.1124045 1 square yard = 9 sq ft sq yd 0.83613 m2 1.19599 1 long ton-weight l tn wt 9.964015 kN 0.1003611 1 acre = 4 roods 4046.8 -

THE SOLUBILITY of GASES in LIQUIDS Introductory Information C
THE SOLUBILITY OF GASES IN LIQUIDS Introductory Information C. L. Young, R. Battino, and H. L. Clever INTRODUCTION The Solubility Data Project aims to make a comprehensive search of the literature for data on the solubility of gases, liquids and solids in liquids. Data of suitable accuracy are compiled into data sheets set out in a uniform format. The data for each system are evaluated and where data of sufficient accuracy are available values are recommended and in some cases a smoothing equation is given to represent the variation of solubility with pressure and/or temperature. A text giving an evaluation and recommended values and the compiled data sheets are published on consecutive pages. The following paper by E. Wilhelm gives a rigorous thermodynamic treatment on the solubility of gases in liquids. DEFINITION OF GAS SOLUBILITY The distinction between vapor-liquid equilibria and the solubility of gases in liquids is arbitrary. It is generally accepted that the equilibrium set up at 300K between a typical gas such as argon and a liquid such as water is gas-liquid solubility whereas the equilibrium set up between hexane and cyclohexane at 350K is an example of vapor-liquid equilibrium. However, the distinction between gas-liquid solubility and vapor-liquid equilibrium is often not so clear. The equilibria set up between methane and propane above the critical temperature of methane and below the criti cal temperature of propane may be classed as vapor-liquid equilibrium or as gas-liquid solubility depending on the particular range of pressure considered and the particular worker concerned. -

Pressure Diffusion Waves in Porous Media
Lawrence Berkeley National Laboratory Lawrence Berkeley National Laboratory Title Pressure diffusion waves in porous media Permalink https://escholarship.org/uc/item/5bh9f6c4 Authors Silin, Dmitry Korneev, Valeri Goloshubin, Gennady Publication Date 2003-04-08 eScholarship.org Powered by the California Digital Library University of California Pressure diffusion waves in porous media Dmitry Silin* and Valeri Korneev, Lawrence Berkeley National Laboratory, Gennady Goloshubin, University of Houston Summary elastic porous medium. Such a model results in a parabolic pressure diffusion equation. Its validity has been Pressure diffusion wave in porous rocks are under confirmed and “canonized”, for instance, in transient consideration. The pressure diffusion mechanism can pressure well test analysis, where it is used as the main tool provide an explanation of the high attenuation of low- since 1930th, see e.g. Earlougher (1977) and Barenblatt et. frequency signals in fluid-saturated rocks. Both single and al., (1990). The basic assumptions of this model make it dual porosity models are considered. In either case, the applicable specifically in the low-frequency range of attenuation coefficient is a function of the frequency. pressure fluctuations. Introduction Theories describing wave propagation in fluid-bearing porous media are usually derived from Biot’s theory of poroelasticity (Biot 1956ab, 1962). However, the observed high attenuation of low-frequency waves (Goloshubin and Korneev, 2000) is not well predicted by this theory. One of possible reasons for difficulties in detecting Biot waves in real rocks is in the limitations imposed by the assumptions underlying Biot’s equations. Biot (1956ab, 1962) derived his main equations characterizing the mechanical motion of elastic porous fluid-saturated rock from the Hamiltonian Principle of Least Action. -

2007 MTS Overview of Manned Underwater Vehicle Activity
P A P E R 2007 MTS Overview of Manned Underwater Vehicle Activity AUTHOR ABSTRACT William Kohnen There are approximately 100 active manned submersibles in operation around the world; Chair, MTS Manned Underwater in this overview we refer to all non-military manned underwater vehicles that are used for Vehicles Committee scientific, research, tourism, and commercial diving applications, as well as personal leisure SEAmagine Hydrospace Corporation craft. The Marine Technology Society committee on Manned Underwater Vehicles (MUV) maintains the only comprehensive database of active submersibles operating around the world and endeavors to continually bring together the international community of manned Introduction submersible operators, manufacturers and industry professionals. The database is maintained he year 2007 did not herald a great through contact with manufacturers, operators and owners through the Manned Submersible number of new manned submersible de- program held yearly at the Underwater Intervention conference. Tployments, although the industry has expe- The most comprehensive and detailed overview of this industry is given during the UI rienced significant momentum. Submersi- conference, and this article cannot cover all developments within the allocated space; there- bles continue to find new applications in fore our focus is on a compendium of activity provided from the most dynamic submersible tourism, science and research, commercial builders, operators and research organizations that contribute to the industry and who share and recreational work; the biggest progress their latest information through the MTS committee. This article presents a short overview coming from the least likely source, namely of submersible activity in 2007, including new submersible construction, operation and the leisure markets. -

Optimal Breathing Gas Mixture in Professional Diving with Multiple Supply
Proceedings of the World Congress on Engineering 2021 WCE 2021, July 7-9, 2021, London, U.K. Optimal Breathing Gas Mixture in Professional Diving with Multiple Supply Orhan I. Basaran, Mert Unal compressors and cylinders, it was limited to surface air Abstract— Professional diving existed since antiquities when supply lines. In 1978, Fleuss introduced the first closed divers collected resources from the bottom of the seas and circuit oxygen breathing apparatus which removed carbon lakes. With technological advancements in the recent century, dioxide from the exhaled gas and did not form bubbles professional diving activities also increased significantly. underwater. In 1943, Cousteau and Gangan designed the Diving has many adverse effects on human physiology which first proper demand-regulated air supply from compressed are widely investigated in order to make dives safer. In this air cylinders worn on the back. The scuba equipment with study, we focus on optimizing the breathing gas mixture minimizing the dive costs while ensuring the safety of the the high-pressure regulator on the cylinder and a single hose divers. The methods proposed in this paper are purely to a demand valve was invented in Australia and marketed theoretical and divers should always have appropriate training by Ted Eldred in the early 1950s [1]. and certificates. Also, divers should never perform dives With the use of Siebe dress, the first cases of decompression without consulting professionals and medical doctors with expertise in related fields. sickness began to be documented. Haldane conducted several experiments on animal and human subjects in Index Terms—-professional diving; breathing gas compression chambers to investigate the causes of this optimization; dive profile optimization sickness and how it can be prevented. -

What Is High Blood Pressure?
ANSWERS Lifestyle + Risk Reduction by heart High Blood Pressure BLOOD PRESSURE SYSTOLIC mm Hg DIASTOLIC mm Hg What is CATEGORY (upper number) (lower number) High Blood NORMAL LESS THAN 120 and LESS THAN 80 ELEVATED 120-129 and LESS THAN 80 Pressure? HIGH BLOOD PRESSURE 130-139 or 80-89 (HYPERTENSION) Blood pressure is the force of blood STAGE 1 pushing against blood vessel walls. It’s measured in millimeters of HIGH BLOOD PRESSURE 140 OR HIGHER or 90 OR HIGHER mercury (mm Hg). (HYPERTENSION) STAGE 2 High blood pressure (HBP) means HYPERTENSIVE the pressure in your arteries is higher CRISIS HIGHER THAN 180 and/ HIGHER THAN 120 than it should be. Another name for (consult your doctor or immediately) high blood pressure is hypertension. Blood pressure is written as two numbers, such as 112/78 mm Hg. The top, or larger, number (called Am I at higher risk of developing HBP? systolic pressure) is the pressure when the heart There are risk factors that increase your chances of developing HBP. Some you can control, and some you can’t. beats. The bottom, or smaller, number (called diastolic pressure) is the pressure when the heart Those that can be controlled are: rests between beats. • Cigarette smoking and exposure to secondhand smoke • Diabetes Normal blood pressure is below 120/80 mm Hg. • Being obese or overweight If you’re an adult and your systolic pressure is 120 to • High cholesterol 129, and your diastolic pressure is less than 80, you have elevated blood pressure. High blood pressure • Unhealthy diet (high in sodium, low in potassium, and drinking too much alcohol) is a systolic pressure of 130 or higher,or a diastolic pressure of 80 or higher, that stays high over time. -

Ocean Storage
277 6 Ocean storage Coordinating Lead Authors Ken Caldeira (United States), Makoto Akai (Japan) Lead Authors Peter Brewer (United States), Baixin Chen (China), Peter Haugan (Norway), Toru Iwama (Japan), Paul Johnston (United Kingdom), Haroon Kheshgi (United States), Qingquan Li (China), Takashi Ohsumi (Japan), Hans Pörtner (Germany), Chris Sabine (United States), Yoshihisa Shirayama (Japan), Jolyon Thomson (United Kingdom) Contributing Authors Jim Barry (United States), Lara Hansen (United States) Review Editors Brad De Young (Canada), Fortunat Joos (Switzerland) 278 IPCC Special Report on Carbon dioxide Capture and Storage Contents EXECUTIVE SUMMARY 279 6.7 Environmental impacts, risks, and risk management 298 6.1 Introduction and background 279 6.7.1 Introduction to biological impacts and risk 298 6.1.1 Intentional storage of CO2 in the ocean 279 6.7.2 Physiological effects of CO2 301 6.1.2 Relevant background in physical and chemical 6.7.3 From physiological mechanisms to ecosystems 305 oceanography 281 6.7.4 Biological consequences for water column release scenarios 306 6.2 Approaches to release CO2 into the ocean 282 6.7.5 Biological consequences associated with CO2 6.2.1 Approaches to releasing CO2 that has been captured, lakes 307 compressed, and transported into the ocean 282 6.7.6 Contaminants in CO2 streams 307 6.2.2 CO2 storage by dissolution of carbonate minerals 290 6.7.7 Risk management 307 6.2.3 Other ocean storage approaches 291 6.7.8 Social aspects; public and stakeholder perception 307 6.3 Capacity and fractions retained -

THE SOLUBILITY of GASES in LIQUIDS INTRODUCTION the Solubility Data Project Aims to Make a Comprehensive Search of the Lit- Erat
THE SOLUBILITY OF GASES IN LIQUIDS R. Battino, H. L. Clever and C. L. Young INTRODUCTION The Solubility Data Project aims to make a comprehensive search of the lit erature for data on the solubility of gases, liquids and solids in liquids. Data of suitable accuracy are compiled into data sheets set out in a uni form format. The data for each system are evaluated and where data of suf ficient accuracy are available values recommended and in some cases a smoothing equation suggested to represent the variation of solubility with pressure and/or temperature. A text giving an evaluation and recommended values and the compiled data sheets are pUblished on consecutive pages. DEFINITION OF GAS SOLUBILITY The distinction between vapor-liquid equilibria and the solUbility of gases in liquids is arbitrary. It is generally accepted that the equilibrium set up at 300K between a typical gas such as argon and a liquid such as water is gas liquid solubility whereas the equilibrium set up between hexane and cyclohexane at 350K is an example of vapor-liquid equilibrium. However, the distinction between gas-liquid solUbility and vapor-liquid equilibrium is often not so clear. The equilibria set up between methane and propane above the critical temperature of methane and below the critical temperature of propane may be classed as vapor-liquid equilibrium or as gas-liquid solu bility depending on the particular range of pressure considered and the par ticular worker concerned. The difficulty partly stems from our inability to rigorously distinguish between a gas, a vapor, and a liquid, which has been discussed in numerous textbooks. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S.