Pressure, Its Units of Measure and Pressure References
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

11 Fluid Statics
CHAPTER 11 | FLUID STATICS 357 11 FLUID STATICS Figure 11.1 The fluid essential to all life has a beauty of its own. It also helps support the weight of this swimmer. (credit: Terren, Wikimedia Commons) Learning Objectives 11.1. What Is a Fluid? • State the common phases of matter. • Explain the physical characteristics of solids, liquids, and gases. • Describe the arrangement of atoms in solids, liquids, and gases. 11.2. Density • Define density. • Calculate the mass of a reservoir from its density. • Compare and contrast the densities of various substances. 11.3. Pressure • Define pressure. • Explain the relationship between pressure and force. • Calculate force given pressure and area. 11.4. Variation of Pressure with Depth in a Fluid • Define pressure in terms of weight. • Explain the variation of pressure with depth in a fluid. • Calculate density given pressure and altitude. 11.5. Pascal’s Principle • Define pressure. • State Pascal’s principle. • Understand applications of Pascal’s principle. • Derive relationships between forces in a hydraulic system. 11.6. Gauge Pressure, Absolute Pressure, and Pressure Measurement • Define gauge pressure and absolute pressure. • Understand the working of aneroid and open-tube barometers. 11.7. Archimedes’ Principle • Define buoyant force. • State Archimedes’ principle. • Understand why objects float or sink. • Understand the relationship between density and Archimedes’ principle. 11.8. Cohesion and Adhesion in Liquids: Surface Tension and Capillary Action • Understand cohesive and adhesive forces. • Define surface tension. • Understand capillary action. 11.9. Pressures in the Body • Explain the concept of pressure the in human body. • Explain systolic and diastolic blood pressures. • Describe pressures in the eye, lungs, spinal column, bladder, and skeletal system. -
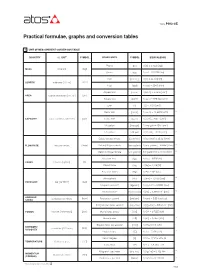
Practical Formulae, Graphs and Conversion Tables
Table P003-4/E Practical formulae, graphs and conversion tables 1 UNIT OF MEASUREMENT CONVERSION TABLE QUANTITY S.I. UNIT SYMBOL OTHER UNITS SYMBOL EQUIVALENCE Pound [lb] 1 [lb] = 0,4536 [kg] kilogram [kg] MASS Ounce [oz] 1 [oz] = 0,02335 [kg] Inch [in] or [”] 1 [in] = 25,40 [mm] millimeter [10-3 m] [mm] LENGTH Foot [foot] 1 [foot] = 304,8 [mm] Square inch [sq in] 1 [sq in] = 6,4516 [cm2] -4 2 [cm2] AREA square centimeter [10 m ] Square foot [sq ft] 1 [sq ft] = 929,034 [cm2] Liter [l] 1 [l] = 1000 [cm3] Cubic inch [cu in] 1 [cu in] = 16,3870 [cm3] * cubic centimeter [10-6 m3] [cm3] Cubic foot [cu ft] 1 [cu ft] = 28317 [cm3] CAPACITY UK gallon [Imp gal] 1 [Imp gal] = 4546 [cm3] US gallon [US gal] 1 [US gal] = 3785 [cm3] * Cubic foot per minute [cu ft/min] 1 [cu ft/min] = 28,32 [l/min] liter per minute [l/min] Gallon (UK) per minute [Imp gal/min] 1 [Imp gal/min] = 4,5456 [l/min] * FLOW RATE Gallon (US) per minute [US gal/min] [US gal/min] = 3,7848 [l/min] * Kilogram force [kgf] 1 [kgf] = 9,806 [N] Newton [kgm/s2] [N] FORCE Pound force [lbf] 1 [lbf] = 4,448 [N] Pascal [1 N/m2] [Pa] 1 [Pa] = 10-5 [bar] Atmosphere [atm] 1 [atm] = 1,0132 [bar] * bar [105 N/m2] [bar] PRESSURE 2 2 2 Kilogram force/cm [kgf/cm ] 1 [kgf/cm ] = 0,9806 [bar] 2 2 -2 Pound force/in [lbf /in ] or [psi] 1 [psi] = 6,8948•10 [bar] * ANGULAR revolution per minute [rpm] Radian per second [rad/sec] 1 [rpm] = 9,55 [rad/sec] SPEED -3 Kilogram per meter second [kgf •m/s] 1 [kgf •m/s] = 9,803•10 [kW] kilowatt [1000 Nm/s] [kW] Metric horse power [CV] 1 [CV] = 0,7355 [kW] POWER -

American and BRITISH UNITS of Measurement to SI UNITS
AMERICAN AND BRITISH UNITS OF MEASUREMENT TO SI UNITS UNIT & ABBREVIATION SI UNITS CONVERSION* UNIT & ABBREVIATION SI UNITS CONVERSION* UNITS OF LENGTH UNITS OF MASS 1 inch = 40 lines in 2.54 cm 0.393701 1 grain gr 64.7989 mg 0.0154324 1 mil 25.4 µm 0.03937 1 dram dr 1.77185 g 0.564383 1 line 0.635 mm 1.57480 1 ounce = 16 drams oz 28.3495 g 0.0352739 1 foot = 12 in = 3 hands ft 30.48 cm 0.0328084 1 pound = 16 oz lb 0.453592 kg 2.204622 1 yard = 3 feet = 4 spans yd 0.9144 m 1.09361 1 quarter = 28 lb 12.7006 kg 0.078737 1 fathom = 2 yd fath 1.8288 m 0.546807 1 hundredweight = 112 lb cwt 50.8024 kg 0.0196841 1 rod (perch, pole) rd 5.0292 m 0.198839 1 long hundredweight l cwt 50.8024 kg 0.0196841 1 chain = 100 links ch 20.1168 m 0.0497097 1 short hundredweight sh cwt 45.3592 kg 0.0220462 1 furlong = 220 yd fur 0.201168 km 4.97097 1 ton = 1 long ton tn, l tn 1.016047 t 0.984206 1 mile (Land Mile) mi 1.60934 km 0.62137 1 short ton = 2000 lb sh tn 0.907185 t 1.102311 1 nautical mile (intl.) n mi, NM 1.852 km 0.539957 1 knot (Knoten) kn 1.852 km/h 0.539957 UNITS OF FORCE 1 pound-weight lb wt 4.448221 N 0.2248089 UNITS OF AREA 1 pound-force LB, lbf 4.448221 N 0.2248089 1 square inch sq in 6.4516 cm2 0.155000 1 poundal pdl 0.138255 N 7.23301 1 circular inch 5.0671 cm2 0.197352 1 kilogram-force kgf, kgp 9.80665 N 0.1019716 1 square foot = 144 sq in sq ft 929.03 cm2 1.0764 x 10-4 1 short ton-weight sh tn wt 8.896444 kN 0.1124045 1 square yard = 9 sq ft sq yd 0.83613 m2 1.19599 1 long ton-weight l tn wt 9.964015 kN 0.1003611 1 acre = 4 roods 4046.8 -

Pressure Measuring Instruments
testo-312-2-3-4-P01 21.08.2012 08:49 Seite 1 We measure it. Pressure measuring instruments For gas and water installers testo 312-2 HPA testo 312-3 testo 312-4 BAR °C www.testo.com testo-312-2-3-4-P02 23.11.2011 14:37 Seite 2 testo 312-2 / testo 312-3 We measure it. Pressure meters for gas and water fitters Use the testo 312-2 fine pressure measuring instrument to testo 312-2 check flue gas draught, differential pressure in the combustion chamber compared with ambient pressure testo 312-2, fine pressure measuring or gas flow pressure with high instrument up to 40/200 hPa, DVGW approval, incl. alarm display, battery and resolution. Fine pressures with a resolution of 0.01 hPa can calibration protocol be measured in the range from 0 to 40 hPa. Part no. 0632 0313 DVGW approval according to TRGI for pressure settings and pressure tests on a gas boiler. • Switchable precision range with a high resolution • Alarm display when user-defined limit values are • Compensation of measurement fluctuations caused by exceeded temperature • Clear display with time The versatile pressure measuring instrument testo 312-3 testo 312-3 supports load and gas-rightness tests on gas and water pipelines up to 6000 hPa (6 bar) quickly and reliably. testo 312-3 versatile pressure meter up to Everything you need to inspect gas and water pipe 300/600 hPa, DVGW approval, incl. alarm display, battery and calibration protocol installations: with the electronic pressure measuring instrument testo 312-3, pressure- and gas-tightness can be tested. -

Surface Tension Measurement." Copyright 2000 CRC Press LLC
David B. Thiessen, et. al.. "Surface Tension Measurement." Copyright 2000 CRC Press LLC. <http://www.engnetbase.com>. Surface Tension Measurement 31.1 Mechanics of Fluid Surfaces 31.2 Standard Methods and Instrumentation Capillary Rise Method • Wilhelmy Plate and du Noüy Ring Methods • Maximum Bubble Pressure Method • Pendant Drop and Sessile Drop Methods • Drop Weight or Volume David B. Thiessen Method • Spinning Drop Method California Institute of Technology 31.3 Specialized Methods Dynamic Surface Tension • Surface Viscoelasticity • Kin F. Man Measurements at Extremes of Temperature and Pressure • California Institute of Technology Interfacial Tension The effect of surface tension is observed in many everyday situations. For example, a slowly leaking faucet drips because the force of surface tension allows the water to cling to it until a sufficient mass of water is accumulated to break free. Surface tension can cause a steel needle to “float” on the surface of water although its density is much higher than that of water. The surface of a liquid can be thought of as having a skin that is under tension. A liquid droplet is somewhat analogous to a balloon filled with air. The elastic skin of the balloon contains the air inside at a slightly higher pressure than the surrounding air. The surface of a liquid droplet likewise contains the liquid in the droplet at a pressure that is slightly higher than ambient. A clean liquid surface, however, is not elastic like a rubber skin. The tension in a piece of rubber increases as it is stretched and will eventually rupture. A clean liquid surface can be expanded indefinitely without changing the surface tension. -

Lecture # 04 Pressure Measurement Techniques and Instrumentation
AerE 344 class notes LectureLecture ## 0404 PressurePressure MeasurementMeasurement TechniquesTechniques andand InstrumentationInstrumentation Hui Hu Department of Aerospace Engineering, Iowa State University Ames, Iowa 50011, U.S.A Copyright © by Dr. Hui Hu @ Iowa State University. All Rights Reserved! MeasurementMeasurement TechniquesTechniques forfor ThermalThermal--FluidsFluids StudiesStudies Velocity, temperature, density (concentration), etc.. • Pitot probe • hotwire, hot film Intrusive • thermocouples techniques • etc ... Thermal-Fluids measurement techniques • Laser Doppler Velocimetry (LDV) particle-based • Planar Doppler Velocimetry (PDV) techniques • Particle Image Velocimetry (PIV) • etc… Non-intrusive techniques • Laser Induced Fluorescence (LIF) • Molecular Tagging Velocimetry (MTV) molecule-based • Molecular Tagging Therometry (MTT) techniques • Pressure Sensitive Paint (PSP) • Temperature Sensitive Paint (TSP) • Quantum Dot Imaging • etc … Copyright © by Dr. Hui Hu @ Iowa State University. All Rights Reserved! Pressure measurements • Pressure is defined as the amount of force that presses on a certain area. – The pressure on the surface will increase if you make the force on an area bigger. – Making the area smaller and keeping the force the same also increase the pressure. – Pressure is a scalar F dF P = n = n A dA nˆ dFn dA τˆ Copyright © by Dr. Hui Hu @ Iowa State University. All Rights Reserved! Pressure measurements Pgauge = Pabsolute − Pamb Manometer Copyright © by Dr. Hui Hu @ Iowa State University. All Rights Reserved! -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Pressure Measurement Explained
Pressure measurement explained Rev A1, May 25th, 2018 Sens4Knowledge Sens4 A/S – Nordre Strandvej 119 G – 3150 Hellebaek – Denmark Phone: +45 8844 7044 – Email: [email protected] www.sens4.com Sens4Knowledge Pressure measurement explained Introduction Pressure is defined as the force per area that can be exerted by a liquid, gas or vapor etc. on a given surface. The applied pressure can be measured as absolute, gauge or differential pressure. Pressure can be measured directly by measurement of the applied force or indirectly, e.g. by the measurement of the gas properties. Examples of indirect measurement techniques that are using gas properties are thermal conductivity or ionization of gas molecules. Before mechanical manometers and electronic diaphragm pressure sensors were invented, pressure was measured by liquid manometers with mercury or water. Pressure standards In physical science the symbol for pressure is p and the SI (abbreviation from French Le Système. International d'Unités) unit for measuring pressure is pascal (symbol: Pa). One pascal is the force of one Newton per square meter acting perpendicular on a surface. Other commonly used pressure units for stating the pressure level are psi (pounds per square inch), torr and bar. Use of pressure units have regional and applicational preference: psi is commonly used in the United States, while bar the preferred unit of measure in Europe. In the industrial vacuum community, the preferred pressure unit is torr in the United States, mbar in Europe and pascal in Asia. Unit conversion Pa bar psi torr atm 1 Pa = 1 1×10-5 1.45038×10-4 7.50062×10-3 9.86923×10-6 1 bar = 100,000 1 14.5038 750.062 0.986923 1 psi = 6,894.76 6.89476×10-2 1 51.7149 6.80460×10-2 1 torr = 133.322 1.33322×10-3 1.933768×10-2 1 1.31579×10-3 1 atm (standard) = 1013.25 1.01325 14.6959 760.000 1 According to the International Organization for Standardization the standard ISO 2533:1975 defines the standard atmospheric pressure of 101,325 Pa (1 atm, 1013.25 mbar or 14.6959 psi). -

Common Units
Civil Engineering Hydraulics Pressure and Fluid Statics Leonard: It wouldn't kill us to meet new people. Sheldon: For the record, it could kill us to meet new people. Common Units ¢ In order to be able to discuss and analyze fluid problems we need to be able to understand some fundamental terms commonly used 2 Pressure 1 Common Units ¢ The most used term in hydraulics and fluid mechanics is probably pressure ¢ Pressure is defined as the normal force exerted by a fluid per unit of area l The important part of that definition is the normal (perpendicular) to the unit of area 3 Pressure Common Units ¢ The Pascal is a very small unit of pressure so it is most often encountered with a prefix to allow the numerical values to be easy to display ¢ Common prefixes are the Kilopascal (kPa=103Pa), the Megapascal (MPa=106Pa), and sometimes the Gigapascal (GPa=109Pa) 4 Pressure 2 Common Units ¢ A bar is defined as 105 Pa so a millibar (mbar) is defined as 10-3 bar so the millibar is 102 Pa The word bar finds its origin in the Greek word báros, meaning weight. 5 Pressure Common Units ¢ Standard atmospheric pressure or "the standard atmosphere" (1 atm) is defined as 101.325 kilopascals (kPa). 6 Pressure 3 Common Units ¢ This "standard pressure" is a purely arbitrary representative value for pressure at sea level, and real atmospheric pressures vary from place to place and moment to moment everywhere in the world. 7 Pressure Common Units ¢ Pressure is usually given in reference to some datum l Absolute pressure is given in reference to a system with -

Vacuum Cup Selection Guide As Is True in Most Vacuum Applications, There Is More Than One Correct Answer
Vacuum Cups & Fittings Vacuum Cup Selection Guide As is true in most vacuum applications, there is more than one correct answer. In order to successfully find the best cup(s) and pumps for a specific task, it is helpful to review the guidelines below. Vacuum Cup Sizing Choose the cup size, quantity, material and style based on the size of the object being handled, its weight, orientation, surface temperature, condi- tions and space available to mount the cups. I. Determine the cup size by using the “Vacuum Cup Holding Force Calculation:” FORCE PRESSURE AREA = X "d" 2 OBJECT WEIGHT CONVERT "Hg TO PSI, AREA = (d /4) or AREA = r 2 SAFETY FACTOR * Lbs (Kg) DIVIDE BY 2 = 3.14 2 2 (2Hg" = 1 PSI) in (cm ) PSI (Kpa) Force = Pressure x Area F = the weight of the objects in lbs(kg) multiplied by the safety factor, see below. P = the expected vacuum level in PSI (Kpa) (2Hg" = 1 PSI) A = the area of the Vacuum cup measured by in2 [cm2] Safety Factors: Always include safety factors when calculating lifting capabilities. Safety Factor=4 Safety Factor=2 Horizontal Lift = 2 Vertical Lift = 4 Safety factor of 2 is recommended when Safety factor of 4 is recommended when cup face is in horizontal position. cup face is in a vertical position. Phone: 1-800-848-8788 or 508-359-7200 E-Mail: [email protected] Vacuum Cups & Fittings II. Determine Type of Material to be handled: Non-Porous, Porous, Flexible/Non-Porous Materials being handled in pick & place applications can be grouped into three categories – non-porous, porous and flexible. -

Total Pressure Measurement
Total Pressure Measurement Vacuum Sensors Display and control unit 5 Accessories Contents Introduction Page 5-3 to 5-6 Hot Cathode Ionization Vacuum Sensorss Page 5-7 to 5-14 Cold Cathode Ionization Vacuum Sensors Page 5-15 to 5-17 Heat Loss Vacuum Sensors Page 5-18 to 5-25 Capacitance Diaphragm Sensors Page 5-26 to 5-30 Relative Pressure Sensors Page 5-31 to 5-33 5 Display and Control Units Page 5-34 to 5-39 Contamination Protection Page 5-39 Cables Page 5-40 to 5-41 5-2 www.vacom-vacuum.com Total Pressure Measurement Introduction For all types of vacuum applications VACOM offers reliable total pressure sensors covering a wide pressure range from atmosphere to XHV. This catalogue introduces VACOM’s own, technologically leading products that can be used out-of-the-box or adapted in a customer specific version. The following chapter provides an overview of VACOM-selected sensors for total pressure measurement as well as suitable controllers. Used to determine the absolute pressure in almost every application. For more detailed information about vacuum gauges please do not hesitate to contact our technical support team. Measuring Principles for Total Pressure Instruments and Typical Measurement Ranges Extreme/Ultra high vacuum High vacuum Medium/Rough vacuum Bourdon 5 Heat loss (Pirani, Thermocouple) Membrane (capacitance, piezo) Wide range (ionization and heat loss) Ionization (Hot Cathode Ionization, Cold Cathode Ionization) Custom made (ionization, heat loss) 10-12 10-10 10-8 10-6 10-4 10-2 100 102 mbar Pressure Units 1 Pa = 0.01 mbar -

General Conversion Table in Alphabetical Order
General Conversion Table In Alphabetical Order UNIT x FACTOR = UNIT UNIT x FACTOR = UNIT Acceleration gravity 9.80665 meter/second2 british thermal unit (BTU) 1054.35 watt-seconds Acceleration gravity 32.2 feet/second2 british thermal unit (BTU) 10.544 x 103 ergs Acceleration gravity 9.80665 meter/second2 british thermal unit (BTU) 0.999331 BTU (IST) Acceleration gravity 32.2 feet/second2 BTU/min 0.01758 kilowatts acre 4,046.856 meter2 BTU/min 0.02358 horsepower acre 0.40469 hectare byte 8.000001 bits acre 43,560.0 foot2 calorie, g 0.00397 british thermal unit acre 4,840.0 yard2 calorie, g 0.00116 watt-hour acre 0.00156 mile2 (statute) calorie, g 4184.00 x 103 ergs acre 0.00404686 kilometer2 calorie, g 3.08596 foot pound-force acre 160 rods2 calorie, g 4.184 joules acre feet 1,233.489 meter2 calorie, g 0.000001162 kilowatt-hour acre feet 325,851.0 gallon (US) calorie, g 42664.9 gram-force cm acre feet 1,233.489 meter3 calorie, g/hr 0.00397 btu/hr acre feet 325,851.0 gallon calorie, g/hr 0.0697 watts acre-feet 43560 feet3 candle/cm2 12.566 candle/inch2 acre-feet 102.7901531 meter3 candle/cm2 10000.0 candle/meter2 acre-feet 134.44 yards3 candle/inch2 144.0 candle/foot2 ampere 1 coulombs/second candle power 12.566 lumens ampere 0.0000103638 faradays/second carats 3.0865 grains ampere 2997930000.0 statamperes carats 200.0 milligrams ampere 1000 milliamperes celsius 1.8C°+ 32 fahrenheit ampere/meter 3600 coulombs celsius 273.16 + C° kelvin angstrom 0.0001 microns centimeter 0.39370 inch angstrom 0.1 millimicrons centimeter 0.03281 foot atmosphere