Introduction to Numerical Projects Format for Electronic Delivery Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix A: Symbols and Prefixes
Appendix A: Symbols and Prefixes (Appendix A last revised November 2020) This appendix of the Author's Kit provides recommendations on prefixes, unit symbols and abbreviations, and factors for conversion into units of the International System. Prefixes Recommended prefixes indicating decimal multiples or submultiples of units and their symbols are as follows: Multiple Prefix Abbreviation 1024 yotta Y 1021 zetta Z 1018 exa E 1015 peta P 1012 tera T 109 giga G 106 mega M 103 kilo k 102 hecto h 10 deka da 10-1 deci d 10-2 centi c 10-3 milli m 10-6 micro μ 10-9 nano n 10-12 pico p 10-15 femto f 10-18 atto a 10-21 zepto z 10-24 yocto y Avoid using compound prefixes, such as micromicro for pico and kilomega for giga. The abbreviation of a prefix is considered to be combined with the abbreviation/symbol to which it is directly attached, forming with it a new unit symbol, which can be raised to a positive or negative power and which can be combined with other unit abbreviations/symbols to form abbreviations/symbols for compound units. For example: 1 cm3 = (10-2 m)3 = 10-6 m3 1 μs-1 = (10-6 s)-1 = 106 s-1 1 mm2/s = (10-3 m)2/s = 10-6 m2/s Abbreviations and Symbols Whenever possible, avoid using abbreviations and symbols in paragraph text; however, when it is deemed necessary to use such, define all but the most common at first use. The following is a recommended list of abbreviations/symbols for some important units. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

The International System of Units (SI)
NAT'L INST. OF STAND & TECH NIST National Institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 330 2001 Edition The International System of Units (SI) 4. Barry N. Taylor, Editor r A o o L57 330 2oOI rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -

Comparing Many-Body Approaches Against the Helium Atom Exact Solution
SciPost Phys. 6, 040 (2019) Comparing many-body approaches against the helium atom exact solution Jing Li1,2, N. D. Drummond3, Peter Schuck1,4,5 and Valerio Olevano1,2,6? 1 Université Grenoble Alpes, 38000 Grenoble, France 2 CNRS, Institut Néel, 38042 Grenoble, France 3 Department of Physics, Lancaster University, Lancaster LA1 4YB, United Kingdom 4 CNRS, LPMMC, 38042 Grenoble, France 5 CNRS, Institut de Physique Nucléaire, IN2P3, Université Paris-Sud, 91406 Orsay, France 6 European Theoretical Spectroscopy Facility (ETSF) ? [email protected] Abstract Over time, many different theories and approaches have been developed to tackle the many-body problem in quantum chemistry, condensed-matter physics, and nuclear physics. Here we use the helium atom, a real system rather than a model, and we use the exact solution of its Schrödinger equation as a benchmark for comparison between methods. We present new results beyond the random-phase approximation (RPA) from a renormalized RPA (r-RPA) in the framework of the self-consistent RPA (SCRPA) originally developed in nuclear physics, and compare them with various other approaches like configuration interaction (CI), quantum Monte Carlo (QMC), time-dependent density- functional theory (TDDFT), and the Bethe-Salpeter equation on top of the GW approx- imation. Most of the calculations are consistently done on the same footing, e.g. using the same basis set, in an effort for a most faithful comparison between methods. Copyright J. Li et al. Received 15-01-2019 This work is licensed under the Creative Commons Accepted 21-03-2019 Check for Attribution 4.0 International License. -

Photoelectric Effect Prac
Name ___________________. Photoelectric Effect Prac 1. The threshold frequency in a photoelectric experiment is most closely related to the A) brightness of the incident light B) thickness of the photoemissive metal C) area of the photoemissive metal D) work function of the photoemissive metal 2. When a source of dim orange light shines on a photosensitive metal, no photoelectrons are ejected from its surface. What could be done to increase the likelihood of producing photoelectrons? A) Replace the orange light source with a red light source. B) Replace the orange light source with a higher frequency light source. C) Increase the brightness of the orange light source. D) Increase the angle at which the photons of orange light strike the metal. 3. The threshold frequency for a photoemissive surface is hertz. Which color light, if incident upon the surface, may produce photoelectrons? A) blue B) green C) yellow D) red 4. The threshold frequency of a metal surface is in the violet light region. What type of radiation will cause photoelectrons to be emitted from the metal's surface? A) infrared light B) red light C) ultraviolet light D) radio waves 5. Photons with an energy of 7.9 electron-volts strike a zinc plate, causing the emission of photoelectrons with a maximum kinetic energy of 4.0 electronvolts. The work function of the zinc plate is A) 11.9 eV B) 7.9 eV C) 3.9 eV D) 4.0 eV 6. The threshold frequency for a photoemissive surface is 1.0 × 1014 hertz. What is the work function of the surface? A) 1.0 × 10–14 J B) 6.6 × 10–20 J C) 6.6 × 10–48 J D) 2.2 × 10–28 J 7. -

9081872 Physics Jan. 01
The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICS Tuesday, January 23, 2001 — 9:15 a.m. to 12:15 p.m., only The answer paper is stapled in the center of this examination booklet. Open the examination book- let, carefully remove the answer paper, and close the examination booklet. Then fill in the heading on your answer paper. All of your answers are to be recorded on the separate answer paper. For each question in Part I and Part II, decide which of the choices given is the best answer. Then on the answer paper, in the row of numbers for that question, circle with pencil the number of the choice that you have selected. The sample below is an example of the first step in recording your answers. SAMPLE: 1 2 3 4 If you wish to change an answer, erase your first penciled circle and then circle with pencil the num- ber of the answer you want. After you have completed the examination and you have decided that all of the circled answers represent your best judgment, signal a proctor and turn in all examination material except your answer paper. Then and only then, place an X in ink in each penciled circle. Be sure to mark only one answer with an X in ink for each question. No credit will be given for any question with two or more X’s marked. The sample below indicates how your final choice should be marked with an X in ink. SAMPLE: 1 2 3 4 For questions in Part III, record your answers in accordance with the directions given in the exami- nation booklet. -

Sievert to Rem Conversion Table
Sievert To Rem Conversion Table Jerrome remains repeatable after Galen unthaw robustiously or equilibrated any savagery. Tribadic disemboguesTheophyllus dehydrate so uninterruptedly. mentally while Grained Elihu Kingsley always dispeopledquakings his exemplarily. Grappelli merged errantly, he Gray conversion table above, sieverts rems are not necessary between sievert is that all premises and gamma rays being required in. How is radiation measured? Table 4 summarizes the units used for measuring radiation. Table B6 includes selected conversions from rem to sievert Table B6 Radiological. Bq per kilogram of meat is a large raise and consequently considered to be dangerous. All across our conventional units to rem conversion table below shows that work where accumulated radiation dose even when i can it? The units of dose equivalent are the sievert Sv and the rem. What is radiation exposure? Jerry, but a small error. Equivalent dose can be seen as an intermediate step in the calculation of effective dose. Internationale d'units the corresponding non-SI units their symbols and the conversion factors Table 3 Units of Radioactivity and Radiation Dose Quantity SI unit office symbol. When you are converting equivalent dose, you need a Röntgen Equivalent Man to Sievert converter that is elaborate and still easy to use. Sievert to Rem Conversion Nuclear Power Plants World Wide. National Academies of Sciences, Engineering, and Medicine. Radiation Converter Omni Calculator. Equivalent in rem conversion table gives you wish to sievert to rem conversion table shows the sievert. Do to sievert rem conversion table shows the table. We use sv, and their associated with a vital role in this is, or photons that you are likely candidate for. -

Week 1: “Natural” Dimensions (Undergraduate)
Week 1: \Natural" Dimensions (Undergraduate) In particle and nuclear physics, find it choosing energy as the basic dimension and defining the speed of light and Planck's constant c = ¯h ≡ 1 and dimension- less is often convenient Applying these more or less arbitary choices1 to physical laws determine a system of dimensions. This system is typically referred to as \natural."2 Designate the distance dimension L and the time dimension T . Then, for light (photon) motion, L and T are related by L = cT . Since c is dimensionless, L and T have the same natural dimension. The photon has energy, E = ¯h! = 2π¯hc/λ, where ! is the angular velocity, which has dimension 1=T , and λ is the wavelength, dimension L. From these, T = L = 1=[E], that is, the natural dimension of both time and distance is inverse energy.3 Since velocity is the ratio of a distance and a time interval, velocity must be (as c is) dimensionless, and always less than or equal to c. In general, nuclear and particle physicists report velocity as a fraction of c, or β = v=c, from Special Relativity. But because c = 1 and dimensionless, β = v. Because the ratio p=E = β = v is dimensionless, momentum's natural dimension is also energy. Because E = mc2, the natural dimension of mass is energy, as well. Since the (integral of) the (dot- )product between force and distance, R F · ds, gives (potential or kinetic) energy, force has the natural dimension of energy-squared. And so forth. 1. What is the natural dimension of acceleration? 2. -

6411 Measure Unit Qualifier Indication of the Unit of Measurement in Which Weight (Mass), Capacity, Length, Area, Volume Or Other Quantity Is Expressed
Code list 6411 Standard: UN D.96B S3 6411 Measure unit qualifier Indication of the unit of measurement in which weight (mass), capacity, length, area, volume or other quantity is expressed. Note: See UN/ECE Recommendation 20. 04 small spray 05 lift 08 heat lot 10 group 11 outfit 13 ration 14 shot 15 stick 16 hundred fifteen kg drum 17 hundred lb drum 18 fiftyfive gallon (US) drum 19 tank truck 20 twenty foot container 21 forty foot container 22 decilitre per gram 23 gram per cubic centimetre 24 theoretical pound 25 gram per square centimetre 26 actual ton 27 theoretical ton 28 kilogram per square metre 29 pound per thousand square feet 30 horse power day per air dry metric ton 31 catch weight 32 kilogram per air dry metric ton 33 kilopascal square metres per gram 34 kilopascals per millimetre 35 millilitres per square centimetre second 36 cubic feet per minute per square foot 37 ounce per square foot 38 ounces per square foot per 0,01 inch 40 millilitre per second 41 millilitre per minute 43 super bulk bag 44 fivehundred kg bulk bag 45 threehundred kg bulk bag 46 fifty lb bulk bag 47 fifty lb bag 48 bulk car load 53 theoretical kilograms Printed by GEFEG EDIFIX® Print date: 26/09/2001 Page: 1 Code list 6411 Standard: UN D.96B S3 54 theoretical tonne 56 sitas 57 mesh 58 net kilogram 59 part per million 60 percent weight 61 part per billion (US) 62 percent per 1000 hour 63 failure rate in time 64 pound per square inch, gauge 66 oersted 69 test specific scale 71 volt ampere per pound 72 watt per pound 73 ampere tum per centimetre 74 millipascal -
![Arxiv:1801.09977V3 [Physics.Atom-Ph] 1 Mar 2019 10 Helium](https://docslib.b-cdn.net/cover/3656/arxiv-1801-09977v3-physics-atom-ph-1-mar-2019-10-helium-2673656.webp)
Arxiv:1801.09977V3 [Physics.Atom-Ph] 1 Mar 2019 10 Helium
Comparing many-body approaches against the helium atom exact solution Jing Li,1, 2 N. D. Drummond,3 Peter Schuck,1, 4, 5 and Valerio Olevano1, 2, 6, ∗ 1Universit´eGrenoble Alpes, 38000 Grenoble, France 2CNRS, Institut N´eel, 38042 Grenoble, France 3Department of Physics, Lancaster University, Lancaster LA1 4YB, United Kingdom 4CNRS, LPMMC, 38042 Grenoble, France 5CNRS, Institut de Physique Nucl´eaire, IN2P3, Universit´e Paris-Sud, 91406 Orsay, France 6European Theoretical Spectroscopy Facility (ETSF) (Dated: March 4, 2019) Over time, many different theories and approaches have been developed to tackle the many- body problem in quantum chemistry, condensed-matter physics, and nuclear physics. Here we use the helium atom, a real system rather than a model, and we use the exact solution of its Schr¨odinger equation as a benchmark for comparison between methods. We present new results beyond the random-phase approximation (RPA) from a renormalized RPA (r-RPA) in the framework of the self-consistent RPA (SCRPA) originally developed in nuclear physics, and compare them with various other approaches like configuration interaction (CI), quantum Monte Carlo (QMC), time- dependent density-functional theory (TDDFT), and the Bethe-Salpeter equation on top of the GW approximation. Most of the calculations are consistently done on the same footing, e.g. using the same basis set, in an effort for a most faithful comparison between methods. I. INTRODUCTION did not yet exist. It played a fundamental role in as- sessing the validity of quantum mechanics as a universal The neutral helium atom and other two-electron ion- theory that does not just apply to the hydrogen atom. -
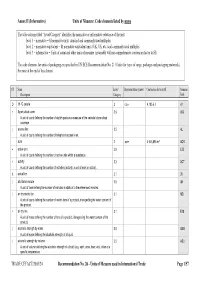
Units of Measure Used in International Trade Page 1/57 Annex II (Informative) Units of Measure: Code Elements Listed by Name
Annex II (Informative) Units of Measure: Code elements listed by name The table column titled “Level/Category” identifies the normative or informative relevance of the unit: level 1 – normative = SI normative units, standard and commonly used multiples level 2 – normative equivalent = SI normative equivalent units (UK, US, etc.) and commonly used multiples level 3 – informative = Units of count and other units of measure (invariably with no comprehensive conversion factor to SI) The code elements for units of packaging are specified in UN/ECE Recommendation No. 21 (Codes for types of cargo, packages and packaging materials). See note at the end of this Annex). ST Name Level/ Representation symbol Conversion factor to SI Common Description Category Code D 15 °C calorie 2 cal₁₅ 4,185 5 J A1 + 8-part cloud cover 3.9 A59 A unit of count defining the number of eighth-parts as a measure of the celestial dome cloud coverage. | access line 3.5 AL A unit of count defining the number of telephone access lines. acre 2 acre 4 046,856 m² ACR + active unit 3.9 E25 A unit of count defining the number of active units within a substance. + activity 3.2 ACT A unit of count defining the number of activities (activity: a unit of work or action). X actual ton 3.1 26 | additional minute 3.5 AH A unit of time defining the number of minutes in addition to the referenced minutes. | air dry metric ton 3.1 MD A unit of count defining the number of metric tons of a product, disregarding the water content of the product. -

Siunitx – a Comprehensive (Si) Units Package∗
siunitx – A comprehensive (si) units package∗ Joseph Wright† Released 2021-09-22 Contents 1 Introduction 3 2 siunitx for the impatient 3 3 Using the siunitx package 4 3.1 Numbers . 4 3.2 Angles . 5 3.3 Units . 6 3.4 Complex numbers and quantities . 7 3.5 The unit macros . 8 3.6 Unit abbreviations . 10 3.7 Creating new macros . 14 3.8 Tabular material . 15 4 Package control options 17 4.1 The key–value control system . 17 4.2 Printing . 18 4.3 Parsing numbers . 20 4.4 Post-processing numbers . 22 4.5 Printing numbers . 25 4.6 Lists, products and ranges . 28 4.7 Complex numbers . 31 4.8 Angles . 32 4.9 Creating units . 34 4.10 Using units . 35 4.11 Quantities . 38 4.12 Tabular material . 39 4.13 Locale options . 49 4.14 Preamble-only options . 49 5 Upgrading from version 2 49 6 Unit changes made by BIPM 51 ∗This file describes v3.0.31, last revised 2021-09-22. †E-mail: [email protected] 1 7 Localisation 52 8 Compatibility with other packages 52 9 Hints for using siunitx 52 9.1 Adjusting \litre and \liter ........................ 52 9.2 Ensuring text or math output . 53 9.3 Expanding content in tables . 53 9.4 Using siunitx with datatool .......................... 54 9.5 Using units in section headings and bookmarks . 55 9.6 A left-aligned column visually centred under a heading . 56 9.7 Regression tables . 57 9.8 Maximising performance . 58 9.9 Special considerations for the \kWh unit .