Quantities, Units and Symbols in Physical Chemistry Third Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Db Math Marco Zennaro Ermanno Pietrosemoli Goals
dB Math Marco Zennaro Ermanno Pietrosemoli Goals ‣ Electromagnetic waves carry power measured in milliwatts. ‣ Decibels (dB) use a relative logarithmic relationship to reduce multiplication to simple addition. ‣ You can simplify common radio calculations by using dBm instead of mW, and dB to represent variations of power. ‣ It is simpler to solve radio calculations in your head by using dB. 2 Power ‣ Any electromagnetic wave carries energy - we can feel that when we enjoy (or suffer from) the warmth of the sun. The amount of energy received in a certain amount of time is called power. ‣ The electric field is measured in V/m (volts per meter), the power contained within it is proportional to the square of the electric field: 2 P ~ E ‣ The unit of power is the watt (W). For wireless work, the milliwatt (mW) is usually a more convenient unit. 3 Gain and Loss ‣ If the amplitude of an electromagnetic wave increases, its power increases. This increase in power is called a gain. ‣ If the amplitude decreases, its power decreases. This decrease in power is called a loss. ‣ When designing communication links, you try to maximize the gains while minimizing any losses. 4 Intro to dB ‣ Decibels are a relative measurement unit unlike the absolute measurement of milliwatts. ‣ The decibel (dB) is 10 times the decimal logarithm of the ratio between two values of a variable. The calculation of decibels uses a logarithm to allow very large or very small relations to be represented with a conveniently small number. ‣ On the logarithmic scale, the reference cannot be zero because the log of zero does not exist! 5 Why do we use dB? ‣ Power does not fade in a linear manner, but inversely as the square of the distance. -
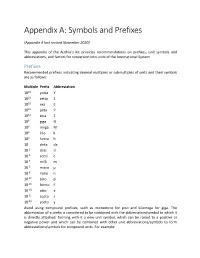
Appendix A: Symbols and Prefixes
Appendix A: Symbols and Prefixes (Appendix A last revised November 2020) This appendix of the Author's Kit provides recommendations on prefixes, unit symbols and abbreviations, and factors for conversion into units of the International System. Prefixes Recommended prefixes indicating decimal multiples or submultiples of units and their symbols are as follows: Multiple Prefix Abbreviation 1024 yotta Y 1021 zetta Z 1018 exa E 1015 peta P 1012 tera T 109 giga G 106 mega M 103 kilo k 102 hecto h 10 deka da 10-1 deci d 10-2 centi c 10-3 milli m 10-6 micro μ 10-9 nano n 10-12 pico p 10-15 femto f 10-18 atto a 10-21 zepto z 10-24 yocto y Avoid using compound prefixes, such as micromicro for pico and kilomega for giga. The abbreviation of a prefix is considered to be combined with the abbreviation/symbol to which it is directly attached, forming with it a new unit symbol, which can be raised to a positive or negative power and which can be combined with other unit abbreviations/symbols to form abbreviations/symbols for compound units. For example: 1 cm3 = (10-2 m)3 = 10-6 m3 1 μs-1 = (10-6 s)-1 = 106 s-1 1 mm2/s = (10-3 m)2/s = 10-6 m2/s Abbreviations and Symbols Whenever possible, avoid using abbreviations and symbols in paragraph text; however, when it is deemed necessary to use such, define all but the most common at first use. The following is a recommended list of abbreviations/symbols for some important units. -

Quantity Symbol Value One Astronomical Unit 1 AU 1.50 × 10
Quantity Symbol Value One Astronomical Unit 1 AU 1:50 × 1011 m Speed of Light c 3:0 × 108 m=s One parsec 1 pc 3.26 Light Years One year 1 y ' π × 107 s One Light Year 1 ly 9:5 × 1015 m 6 Radius of Earth RE 6:4 × 10 m Radius of Sun R 6:95 × 108 m Gravitational Constant G 6:67 × 10−11m3=(kg s3) Part I. 1. Describe qualitatively the funny way that the planets move in the sky relative to the stars. Give a qualitative explanation as to why they move this way. 2. Draw a set of pictures approximately to scale showing the sun, the earth, the moon, α-centauri, and the milky way and the spacing between these objects. Give an ap- proximate size for all the objects you draw (for example example next to the moon put Rmoon ∼ 1700 km) and the distances between the objects that you draw. Indicate many times is one picture magnified relative to another. Important: More important than the size of these objects is the relative distance between these objects. Thus for instance you may wish to show the sun and the earth on the same graph, with the circles for the sun and the earth having the correct ratios relative to to the spacing between the sun and the earth. 3. A common unit of distance in Astronomy is a parsec. 1 pc ' 3:1 × 1016m ' 3:3 ly (a) Explain how such a curious unit of measure came to be defined. Why is it called parsec? (b) What is the distance to the nearest stars and how was this distance measured? 4. -

In Physical Chemistry
INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY Physical and Biophysical Chemistry Division IUPAC Vc^llclllul LICb . U 111 Lb cillCl Oy 111 DOlo in Physical Chemistry Third Edition Prepared for publication by E. Richard Cohen Tomislav Cvitas Jeremy G. Frey BcTt.il Holmstrom Kozo Kuchitsu R,oberto Marquardt Ian Mills Franco Pavese Martin Quack Jiirgen Stohner Herbert L. Strauss Michio Takanii Anders J Thor The first and second editions were prepared for publication by Ian Mills Tomislav Cvitas Klaus Homann Nikola Kallay Kozo Kuchitsu IUPAC 2007 RSC Publishing CONTENTS v PREFACE ix HISTORICAL INTRODUCTION xi 1 PHYSICAL QUANTITIES AND UNITS 1 1.1 Physical quantities and quantity calculus 3 1.2 Base quantities and derived quantities 4 1.3 Symbols for physical quantities and units 5 1.3.1 General rules for symbols for quantities 5 1.3.2 General rules for symbols for units 5 1.4 Use of the words "extensive", "intensive", "specific", and "molar" 6 1.5 Products and quotients of physical quantities and units 7 1.6 The use of italic and Roman fonts for symbols in scientific publications 7 2 TABLES OF PHYSICAL QUANTITIES 11 2.1 Space and time 13 2.2 Classical mechanics 14 2.3 Electricity and magnetism 16 2.4 Quantum mechanics and quantum chemistry 18 2.4.1 Ab initio Hartree-Fock self-consistent field theory (ab initio SCF) 20 2.4.2 Hartree-Fock-Roothaan SCF theory, using molecular orbitals expanded as linear combinations of atomic-orbital basis . functions (LCAO-MO theory) . 21 2.5 Atoms and molecules 22 2.6 Spectroscopy 25 2.6.1 Symbols for angular -

Units, Conversations, and Scaling Giving Numbers Meaning and Context Author: Meagan White; Sean S
Units, Conversations, and Scaling Giving numbers meaning and context Author: Meagan White; Sean S. Lindsay Version 1.3 created August 2019 Learning Goals In this lab, students will • Learn about the metric system • Learn about the units used in science and astronomy • Learn about the units used to measure angles • Learn the two sky coordinate systems used by astronomers • Learn how to perform unit conversions • Learn how to use a spreadsheet for repeated calculations • Measure lengths to collect data used in next week’s lab: Scienctific Measurement Materials • Calculator • Microsoft Excel • String as a crude length measurement tool Pre-lab Questions 1. What is the celestial analog of latitude and longitude? 2. What quantity do the following metric prefixes indicate: Giga-, Mega-, kilo-, centi-, milli-, µicro-, and nano? 3. How many degrees is a Right Ascension “hour”; How many degrees are in an arcmin? Arcsec? 4. Use the “train-track” method to convert 20 ft to inches. 1. Background In any science class, including astronomy, there are important skills and concepts that students will need to use and understand before engaging in experiments and other lab exercises. A broad list of fundamental skills developed in today’s exercise includes: • Scientific units used throughout the international science community, as well as units specific to astronomy. • Unit conversion methods to convert between units for calculations, communication, and conceptualization. • Angular measurements and their use in astronomy. How they are measured, and the angular measurements specific to astronomy. This is important for astronomers coordinate systems, i.e., equatorial coordinates on the celestial sphere. • Familiarity with spreadsheet programs, such as Microsoft Excel, Google Sheets, or OpenOffice. -

SF Chemical Kinetics. Microscopic Theories of Chemical Reaction Kinetics
SF Chemical Kinetics. Lecture 5. Microscopic theory of chemical reaction kinetics. Microscopic theories of chemical reaction kinetics. • A basic aim is to calculate the rate constant for a chemical reaction from first principles using fundamental physics. • Any microscopic level theory of chemical reaction kinetics must result in the derivation of an expression for the rate constant that is consistent with the empirical Arrhenius equation. • A microscopic model should furthermore provide a reasonable interpretation of the pre-exponential factor A and the activation energy EA in the Arrhenius equation. • We will examine two microscopic models for chemical reactions : – The collision theory. – The activated complex theory. • The main emphasis will be on gas phase bimolecular reactions since reactions in the gas phase are the most simple reaction types. 1 References for Microscopic Theory of Reaction Rates. • Effect of temperature on reaction rate. – Burrows et al Chemistry3, Section 8. 7, pp. 383-389. • Collision Theory/ Activated Complex Theory. – Burrows et al Chemistry3, Section 8.8, pp.390-395. – Atkins, de Paula, Physical Chemistry 9th edition, Chapter 22, Reaction Dynamics. Section. 22.1, pp.832-838. – Atkins, de Paula, Physical Chemistry 9th edition, Chapter 22, Section.22.4-22.5, pp. 843-850. Collision theory of bimolecular gas phase reactions. • We focus attention on gas phase reactions and assume that chemical reactivity is due to collisions between molecules. • The theoretical approach is based on the kinetic theory of gases. • Molecules are assumed to be hard structureless spheres. Hence the model neglects the discrete chemical structure of an individual molecule. This assumption is unrealistic. • We also assume that no interaction between molecules until contact. -

Dimensional Analysis and the Theory of Natural Units
LIBRARY TECHNICAL REPORT SECTION SCHOOL NAVAL POSTGRADUATE MONTEREY, CALIFORNIA 93940 NPS-57Gn71101A NAVAL POSTGRADUATE SCHOOL Monterey, California DIMENSIONAL ANALYSIS AND THE THEORY OF NATURAL UNITS "by T. H. Gawain, D.Sc. October 1971 lllp FEDDOCS public This document has been approved for D 208.14/2:NPS-57GN71101A unlimited release and sale; i^ distribution is NAVAL POSTGRADUATE SCHOOL Monterey, California Rear Admiral A. S. Goodfellow, Jr., USN M. U. Clauser Superintendent Provost ABSTRACT: This monograph has been prepared as a text on dimensional analysis for students of Aeronautics at this School. It develops the subject from a viewpoint which is inadequately treated in most standard texts hut which the author's experience has shown to be valuable to students and professionals alike. The analysis treats two types of consistent units, namely, fixed units and natural units. Fixed units include those encountered in the various familiar English and metric systems. Natural units are not fixed in magnitude once and for all but depend on certain physical reference parameters which change with the problem under consideration. Detailed rules are given for the orderly choice of such dimensional reference parameters and for their use in various applications. It is shown that when transformed into natural units, all physical quantities are reduced to dimensionless form. The dimension- less parameters of the well known Pi Theorem are shown to be in this category. An important corollary is proved, namely that any valid physical equation remains valid if all dimensional quantities in the equation be replaced by their dimensionless counterparts in any consistent system of natural units. -

Department of Chemistry
07 Sept 2020 2020–2021 Module Outlines MSc Chemistry MSc Chemistry with Medicinal Chemistry Please note: This handbook contains outlines for the modules comprise the lecture courses for all the MSc Chemistry and Chemistry with Medicinal Chemistry courses. The outline is also posted on Moodle for each lecture module. If there are any updates to these published outlines, this will be posted on the Level 4 Moodle, showing the date, to indicate that there has been an update. PGT CLASS HEAD: Dr Stephen Sproules, Room A5-14 Phone (direct):0141-330-3719 email: [email protected] LEVEL 4 CLASS HEAD: Dr Linnea Soler, Room A4-36 Phone (direct):0141-330-5345 email: [email protected] COURSE SECRETARY: Mrs Susan Lumgair, Room A4-30 (Teaching Office) Phone: 0141-330-3243 email: [email protected] TEACHING ADMINISTRATOR: Ms Angela Woolton, Room A4-27 (near Teaching Office) Phone: 0141-330-7704 email: [email protected] Module Topics for Lecture Courses The Course Information Table below shows, for each of the courses (e.g. Organic, Inorganic, etc), the lecture modules and associated lecturer. Chemistry with Medicinal Chemistry students take modules marked # in place of modules marked ¶. Organic Organic Course Modules Lecturer Chem (o) o1 Pericyclic Reactions Dr Sutherland o2 Heterocyclic Systems Dr Boyer o3 Advanced Organic Synthesis Dr Prunet o4 Polymer Chemistry Dr Schmidt o5m Asymmetric Synthesis Prof Clark o6m Organic Materials Dr Draper Inorganic Inorganic Course Modules Lecturer Chem (i) i1 Metals in Medicine -

The Avogadro Constant to Be Equal to Exactly 6.02214X×10 23 When It Is Expressed in the Unit Mol −1
[august, 2011] redefinition of the mole and the new system of units zoltan mester It is as easy to count atomies as to resolve the propositions of a lover.. As You Like It William Shakespeare 1564-1616 Argentina, Austria-Hungary, Belgium, Brazil, Denmark, France, German Empire, Italy, Peru, Portugal, Russia, Spain, Sweden and Norway, Switzerland, Ottoman Empire, United States and Venezuela 1875 -May 20 1875, BIPM, CGPM and the CIPM was established, and a three- dimensional mechanical unit system was setup with the base units metre, kilogram, and second. -1901 Giorgi showed that it is possible to combine the mechanical units of this metre–kilogram–second system with the practical electric units to form a single coherent four-dimensional system -In 1921 Consultative Committee for Electricity (CCE, now CCEM) -by the 7th CGPM in 1927. The CCE to proposed, in 1939, the adoption of a four-dimensional system based on the metre, kilogram, second, and ampere, the MKSA system, a proposal approved by the ClPM in 1946. -In 1954, the 10th CGPM, the introduction of the ampere, the kelvin and the candela as base units -in 1960, 11th CGPM gave the name International System of Units, with the abbreviation SI. -in 1970, the 14 th CGMP introduced mole as a unit of amount of substance to the SI 1960 Dalton publishes first set of atomic weights and symbols in 1805 . John Dalton(1766-1844) Dalton publishes first set of atomic weights and symbols in 1805 . John Dalton(1766-1844) Much improved atomic weight estimates, oxygen = 100 . Jöns Jacob Berzelius (1779–1848) Further improved atomic weight estimates . -

Decibels, Phons, and Sones
Decibels, Phons, and Sones The rate at which sound energy reaches a Table 1: deciBel Ratings of Several Sounds given cross-sectional area is known as the Sound Source Intensity deciBel sound intensity. There is an abnormally Weakest Sound Heard 1 x 10-12 W/m2 0.0 large range of intensities over which Rustling Leaves 1 x 10-11 W/m2 10.0 humans can hear. Given the large range, it Quiet Library 1 x 10-9 W/m2 30.0 is common to express the sound intensity Average Home 1 x 10-7 W/m2 50.0 using a logarithmic scale known as the Normal Conversation 1 x 10-6 W/m2 60.0 decibel scale. By measuring the intensity -4 2 level of a given sound with a meter, the Phone Dial Tone 1 x 10 W/m 80.0 -3 2 deciBel rating can be determined. Truck Traffic 1 x 10 W/m 90.0 Intensity values and decibel ratings for Chainsaw, 1 m away 1 x 10-1 W/m2 110.0 several sound sources listed in Table 1. The decibel scale and the intensity values it is based on is an objective measure of a sound. While intensities and deciBels (dB) are measurable, the loudness of a sound is subjective. Sound loudness varies from person to person. Furthermore, sounds with equal intensities but different frequencies are perceived by the same person to have unequal loudness. For instance, a 60 dB sound with a frequency of 1000 Hz sounds louder than a 60 dB sound with a frequency of 500 Hz. -

Chapter 20 Electrochemistry
Chapter 20 Electrochemistry Learning goals and key skills: Identify oxidation, reduction, oxidizing agent, and reducing agent in a chemical equation Complete and balance redox equations using the method of half-reactions. Sketch a voltaic cell and identify its cathode, anode, and the directions in which electrons and ions move. o Calculate standard emfs (cell potentials), E cell, from standard reduction potentials. Use reduction potentials to predict whether a redox reaction is spontaneous. o o Relate E cell to DG and equilibrium constants. Calculate emf under nonstandard conditions. Identify the components of common batteries. Describe the construction of a lithium-ion battery and explain how it works. Describe the construction of a fuel cell and explain how it generates electrical energy. Explain how corrosion occurs and how it is prevented by cathodic protection. Describe the reactions in electrolytic cells. Relate the amounts of products and reactants in redox reactions to electrical charge. Electrochemistry Electrochemistry is the study of the relationships between electricity and chemical reactions. • It includes the study of both spontaneous and nonspontaneous processes. 1 Redox reactions: assigning oxidation numbers Oxidation numbers help keep track of what species loses electrons and what species gains them. • An element is oxidized when the oxidation number increases • An element is reduced when the oxidation number decreases • an oxidizing agent causes another element to be oxidized • a reducing agent causes another element to be reduced. Assigning oxidation numbers (sect. 4.4) 1. Elemental form, each atom has ox. # = 0. Zn O2 O3 I2 S8 P4 2. Simple ions, = charge on ion. -1 for Cl-, +2 for Mg2+ 3. -

Page 1 of 29 Dalton Transactions
Dalton Transactions Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/dalton Page 1 of 29 Dalton Transactions Core-level photoemission spectra of Mo 0.3 Cu 0.7 Sr 2ErCu 2Oy, a superconducting perovskite derivative. Unconventional structure/property relations a, b b b a Sourav Marik , Christine Labrugere , O.Toulemonde , Emilio Morán , M. A. Alario- Manuscript Franco a,* aDpto. Química Inorgánica, Facultad de CC.Químicas, Universidad Complutense de Madrid, 28040- Madrid (Spain) bCNRS, Université de Bordeaux, ICMCB, 87 avenue du Dr. A. Schweitzer, Pessac, F-33608, France