Introduction to Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemistry Grade Level 10 Units 1-15
COPPELL ISD SUBJECT YEAR AT A GLANCE GRADE HEMISTRY UNITS C LEVEL 1-15 10 Program Transfer Goals ● Ask questions, recognize and define problems, and propose solutions. ● Safely and ethically collect, analyze, and evaluate appropriate data. ● Utilize, create, and analyze models to understand the world. ● Make valid claims and informed decisions based on scientific evidence. ● Effectively communicate scientific reasoning to a target audience. PACING 1st 9 Weeks 2nd 9 Weeks 3rd 9 Weeks 4th 9 Weeks Unit 1 Unit 2 Unit 3 Unit 4 Unit 5 Unit 6 Unit Unit Unit Unit Unit Unit Unit Unit Unit 7 8 9 10 11 12 13 14 15 1.5 wks 2 wks 1.5 wks 2 wks 3 wks 5.5 wks 1.5 2 2.5 2 wks 2 2 2 wks 1.5 1.5 wks wks wks wks wks wks wks Assurances for a Guaranteed and Viable Curriculum Adherence to this scope and sequence affords every member of the learning community clarity on the knowledge and skills on which each learner should demonstrate proficiency. In order to deliver a guaranteed and viable curriculum, our team commits to and ensures the following understandings: Shared Accountability: Responding -

Neutron Stars
Chandra X-Ray Observatory X-Ray Astronomy Field Guide Neutron Stars Ordinary matter, or the stuff we and everything around us is made of, consists largely of empty space. Even a rock is mostly empty space. This is because matter is made of atoms. An atom is a cloud of electrons orbiting around a nucleus composed of protons and neutrons. The nucleus contains more than 99.9 percent of the mass of an atom, yet it has a diameter of only 1/100,000 that of the electron cloud. The electrons themselves take up little space, but the pattern of their orbit defines the size of the atom, which is therefore 99.9999999999999% Chandra Image of Vela Pulsar open space! (NASA/PSU/G.Pavlov et al. What we perceive as painfully solid when we bump against a rock is really a hurly-burly of electrons moving through empty space so fast that we can't see—or feel—the emptiness. What would matter look like if it weren't empty, if we could crush the electron cloud down to the size of the nucleus? Suppose we could generate a force strong enough to crush all the emptiness out of a rock roughly the size of a football stadium. The rock would be squeezed down to the size of a grain of sand and would still weigh 4 million tons! Such extreme forces occur in nature when the central part of a massive star collapses to form a neutron star. The atoms are crushed completely, and the electrons are jammed inside the protons to form a star composed almost entirely of neutrons. -

The Practice of Chemistry Education (Paper)
CHEMISTRY EDUCATION: THE PRACTICE OF CHEMISTRY EDUCATION RESEARCH AND PRACTICE (PAPER) 2004, Vol. 5, No. 1, pp. 69-87 Concept teaching and learning/ History and philosophy of science (HPS) Juan QUÍLEZ IES José Ballester, Departamento de Física y Química, Valencia (Spain) A HISTORICAL APPROACH TO THE DEVELOPMENT OF CHEMICAL EQUILIBRIUM THROUGH THE EVOLUTION OF THE AFFINITY CONCEPT: SOME EDUCATIONAL SUGGESTIONS Received 20 September 2003; revised 11 February 2004; in final form/accepted 20 February 2004 ABSTRACT: Three basic ideas should be considered when teaching and learning chemical equilibrium: incomplete reaction, reversibility and dynamics. In this study, we concentrate on how these three ideas have eventually defined the chemical equilibrium concept. To this end, we analyse the contexts of scientific inquiry that have allowed the growth of chemical equilibrium from the first ideas of chemical affinity. At the beginning of the 18th century, chemists began the construction of different affinity tables, based on the concept of elective affinities. Berthollet reworked this idea, considering that the amount of the substances involved in a reaction was a key factor accounting for the chemical forces. Guldberg and Waage attempted to measure those forces, formulating the first affinity mathematical equations. Finally, the first ideas providing a molecular interpretation of the macroscopic properties of equilibrium reactions were presented. The historical approach of the first key ideas may serve as a basis for an appropriate sequencing of -

General Inorganic Chemistry
General Inorganic Chemistry Pre DP Chemistry Period 1 • Teacher: Annika Nyberg • [email protected] • Urgent messages via Wilma! Klicka här för att ändra format på underrubrik i bakgrunden • Course book: • CliffsNotes: Chemistry Quick Review http://www.chem1.com/acad/webtext/virtualtextbook.html Content • Introduction • The Structure of Matter (Chapter 1) • The Atom (Chapter 2 and 3) • Chemical bonding (Chapter 5) • The Mole (Chapter 2) • Solutions (Chapter 9) • Acids and bases (Chapter 10) • Quiz • Revision • EXAM 9.00-11.45 Assessment Exam: 80 % Quiz: 20% + practical work, activity and absences 1. Chemistry: a Science for the twenty-first century ● Chemistry has ancient roots, but is now a modern and active, evolving science. ● Chemistry is often called the central science, because a basic knowledge of chemistry is essential for students in biology, physics, geology and many other subjects. https://www.youtube.com/watch?v=tTlnrhiadnI ● Chemical research and development has provided us with new substances with specific properties. These substances have improved the quality of our lives. Health and medicine vaccines sanitation systems antibiotics anesthesia and all other drugs Energy new alternative energy sources (e.g. solar energy to electric energy, nuclear fission) electric cars with long lasting batteries Environment greenhouse gases acid rain and smog Materials and Technology ● polymers (rubber and nylon), ceramics (cookware), liquid crystals (electronic displays), adhesives (Post-It notes), coatings (latex-paint), silicon chips (computers) Food and Agriculture substances for biotechnology ● The purpose of this course is to make you understand how chemists see the world. ● In other words, if you see one thing (in the macroscopic world) you think another (visualize the particles and events in the microscopic world). -

Basic Chemistry
CH 2- THE CHEMISTRY OF LIFE Atoms . The study of chemistry begins with the basic unit of matter, the atom. The Greek philosopher Democritus called the smallest fragment of matter the atom, from the Greek word atomos. Atoms (cont.) . Placed side by side, 100 million atoms would make a row only about 1 centimeter long. Atoms contain subatomic particles that are even smaller. Atoms (cont.) . What three subatomic particles make up atoms? Atoms (cont.) . The subatomic particles that make up atoms are . protons . neutrons . electrons Atoms (cont.) . Smallest property of an element that still has the properties of that element . “The building blocks of matter” . Atoms are made of smaller (subatomic) particles arranged in a particular way . p+ (proton) . n° (neutron) . e- (electron) Atoms (cont.) . Protons and neutrons have about the same mass. Protons are positively charged particles (+). Neutrons carry no charge (◦). Strong forces bind protons and neutrons together to form the nucleus, which is at the center of the atom. Atoms (cont.) . The electron is a negatively charged particle (−) with 1/1840 the mass of a proton. Electrons are in constant motion in the space surrounding the nucleus (e- cloud). Atoms (cont.) . The subatomic particles in a helium atom. Atoms (cont.) • Electrons are attracted to the positively charged nucleus but remain outside the nucleus because of the energy of their motion. • Because atoms have equal numbers of electrons and protons, and because these subatomic particles have equal but opposite charges, atoms are neutral. Atoms (cont.) . Atomic number- # of p+ AND electrons in an atom . Mass number- total # of p+ + n° in an atom . -

This Ubiquitous Carbon…
Engineering Physics Department Presents Dr. Cristian Contescu Senior Research Staff, Materials Science and Technology Division Oak Ridge National Laboratory This ubiquitous carbon… Abstract: After Stone Age, Bronze Age, and Iron Age, and after the Silicon Age of the informational revolution, the technologies of 21st century are marked by the ubiquitous presence of various forms of carbon allotropes. For a very long time, diamond and graphite were the only known carbon allotropes, but that has changed with the serendipitous discovery of fullerenes, carbon nanotubes, and graphene. Every ten or fifteen years scientists unveil new forms of carbons with new and perplexing properties, while computations suggest that the carbon’s family still has members unknown to us today. At a dramatically accelerated pace, new carbon forms find their place at the leading edge of scientific and technological innovations. At the same time traditional forms of carbon are being used in new and exciting applications that make our life safer, healthier, and more enjoyable. The 21st century may soon be recognized as the Age of Carbon forms. This educational talk will show how carbon, the fourth most abundant element in the Galaxy and the basis of life on Earth, was the engine of most important technological developments throughout the history of civilization. It will emphasize the ability of carbon atoms to generate a variety of mutual combinations and with many other chemical elements. These properties have placed carbon at the core of numerous inventions that define our civilization, while emerging new technologies open a rich path for value-added products in today’s market. -

{TEXTBOOK} Food Products; Their Souce, Chemistry, and Use Ebook
FOOD PRODUCTS; THEIR SOUCE, CHEMISTRY, AND USE Author: E H S 1848-1933 Bailey Number of Pages: 576 pages Published Date: 05 Nov 2015 Publisher: Arkose Press Publication Country: none Language: English ISBN: 9781346054858 DOWNLOAD: FOOD PRODUCTS; THEIR SOUCE, CHEMISTRY, AND USE Food Products; Their Souce, Chemistry, and Use PDF Book Twenty-six internationally respected critics offer their analysis of the issues, their social and ethical implications, and what people are doing in response. He begins with how milk is made in the lactating cell, and proceeds to the basics of cheese making and ice cream manufacture. In works such as the Atlas geografico (1858) and the Atlas pintoresco e historico (1885), he presented independent Mexico to Mexican citizens and the world. Academic Language Mastery: Culture in ContextBy now it's a given: if we're to help our ELLs and SELs access the rigorous demands of today's content standards, we must cultivate the "code" that drives school success: academic language. Other tools, such as antenna modeling software and network analyzer add- ons for PCs and Macs, are addressed, and concluding chapters offer fresh insights into support structures and installation techniques. of the NATO Advanced Study Institute on "Forces in Scanning Probe Methods which was CG-sponsered and organized by the "Forum fUr N anowissenschaften". As is now a tradition, four tutorials were presented on the ?rst day of the meeting. If you want to build your own shipping container home while spending less amount of cash, then keep reading. Successful hair and makeup artist Bernadette Fisers had struggled with her weight for years. -
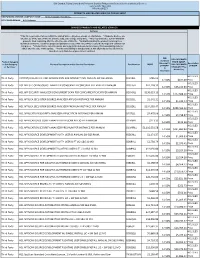
HCL Software's
IBM Branded, Fujistu Branded and Panasonic Branded Products and Related Services and Cloud Services Contract DIR-TSO-3999 PRICING SHEET PRODUCTS AND RELATED SERVICES PRICING SHEET RESPONDING VENDOR COMPANY NAME:____Sirius Computer Solutions_______________ PROPOSED BRAND:__HCL Software__________________________ BRANDED PRODUCTS AND RELATED SERVICES Software * This file is generated for use within the United States. All prices shown are US Dollars. * Products & prices are effective as of the date of this file and are subject to change at any time. * HCL may announce new or withdraw products from marketing after the effective date of this file. * Nothwithstanding the product list and prices identified on this file, customer proposals/quotations issued will reflect HCL's current offerings and commercial list prices. * Product list is not all inclusive and may not include products removed from availability (sale) or added after the date of this update. * Product availability is not guaranteed. Not all products listed below are found on every State/Local government contract. DIR DIR CUSTOMER Customer Product Category PRICE (MSRP- Discount % Description or SubCategory Product Description and/or Service Description Part Number MSRP DIR CUSTOMER off MSRP* of MSRP or Services* DISCOUNT Plus (2 Admin Fee) Decimals) HCL SLED Third Party CNTENT/COLLAB ACC AND WEBSPH PRTL SVR INTRANET PVU ANNUAL SW S&S RNWL E045KLL $786.02 14.50% $677.09 Price HCL SLED Third Party HCL APP SEC OPEN SOURCE ANALYZER CONSCAN PER CONCURRENT EVENT PER ANNUM D20H6LL $41,118.32 -

TEK 8.5C: Periodic Table
Name: Teacher: Pd. Date: TEK 8.5C: Periodic Table TEK 8.5C: Interpret the arrangement of the Periodic Table, including groups and periods, to explain how properties are used to classify elements. Elements and the Periodic Table An element is a substance that cannot be separated into simpler substances by physical or chemical means. An element is already in its simplest form. The smallest piece of an element that still has the properties of that element is called an atom. An element is a pure substance, containing only one kind of atom. The Periodic Table of Elements is a list of all the elements that have been discovered and named, with each element listed in its own element square. Elements are represented on the Periodic Table by a one or two letter symbol, and its name, atomic number and atomic mass. The Periodic Table & Atomic Structure The elements are listed on the Periodic Table in atomic number order, starting at the upper left corner and then moving from the left to right and top to bottom, just as the words of a paragraph are read. The element’s atomic number is based on the number of protons in each atom of that element. In electrically neutral atoms, the atomic number also represents the number of electrons in each atom of that element. For example, the atomic number for neon (Ne) is 10, which means that each atom of neon has 10 protons and 10 electrons. Magnesium (Mg) has an atomic number of 12, which means it has 12 protons and 12 electrons. -

Properties of Carbon the Atomic Element Carbon Has Very Diverse
Properties of Carbon The atomic element carbon has very diverse physical and chemical properties due to the nature of its bonding and atomic arrangement. fig. 1 Allotropes of Carbon Some allotropes of carbon: (a) diamond, (b) graphite, (c) lonsdaleite, (d–f) fullerenes (C60, C540, C70), (g) amorphous carbon, and (h) carbon nanotube. Carbon has several allotropes, or different forms in which it can exist. These allotropes include graphite and diamond, whose properties span a range of extremes. Despite carbon's ability to make 4 bonds and its presence in many compounds, it is highly unreactive under normal conditions. Carbon exists in 2 main isotopes: 12C and 13C. There are many other known isotopes, but they tend to be short-lived and have extremely short half-lives. Allotropes The different forms of a chemical element. Cabon is the chemical element with the symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Carbon has 6 protons and 6 Source URL: https://www.boundless.com/chemistry/nonmetallic-elements/carbon/properties-carbon/ Saylor URL: http://www.saylor.org/courses/chem102#6.1 Attributed to: Boundless www.saylor.org Page 1 of 2 neutrons, and has a standard atomic weight of 12.0107 amu. Its electron configuration is denoted as 1s22s22p2. It is a solid, and sublimes at 3,642 °C. It's oxidation state ranges from 4 to -4, and it has an electronegativity rating of 2.55 on the Pauling scale. Carbon has several allotropes, or different forms in which it exists. -

Centripetal Force Is Balanced by the Circular Motion of the Elctron Causing the Centrifugal Force
STANDARD SC1 b. Construct an argument to support the claim that the proton (and not the neutron or electron) defines the element’s identity. c. Construct an explanation based on scientific evidence of the production of elements heavier than hydrogen by nuclear fusion. d. Construct an explanation that relates the relative abundance of isotopes of a particular element to the atomic mass of the element. First, we quickly review pre-requisite concepts One of the most curious observations with atoms is the fact that there are charged particles inside the atom and there is also constant spinning and Warm-up 1: List the name, charge, mass, and location of the three subatomic circling. How does atom remain stable under these conditions? Remember particles Opposite charges attract each other; Like charges repel each other. Your Particle Location Charge Mass in a.m.u. Task: Read the following information and consult with your teacher as STABILITY OF ATOMS needed, answer Warm-Up tasks 2 and 3 on Page 2. (3) Death spiral does not occur at all! This is because the centripetal force is balanced by the circular motion of the elctron causing the centrifugal force. The centrifugal force is the outward force from the center to the circumference of the circle. Electrons not only spin on their own axis, they are also in a constant circular motion around the nucleus. Despite this terrific movement, electrons are very stable. The stability of electrons mainly comes from the electrostatic forces of attraction between the nucleus and the electrons. The electrostatic forces are also known as Coulombic Forces of Attraction. -

Monitoring Enterprise Collaboration Platform Change and the Building of Digital Transformation Capabilities
Monitoring Enterprise Collaboration Platform Change and the Building of Digital Transformation Capabilities: An Information Infrastructure Perspective by Clara Sabine Nitschke Approved Dissertation thesis for the partial fulfilment of the requirements for a Doctor of Economics and Social Sciences (Dr. rer. pol.) Fachbereich 4: Informatik Universität Koblenz-Landau Chair of PhD Board: Prof. Dr. Ralf Lämmel Chair of PhD Commission: Prof. Dr. Viorica Sofronie-Stokkermans Examiner and Supervisor: Prof. Dr. Susan P. Williams Further Examiners: Prof. Dr. Petra Schubert, Prof. Dr. Catherine A. Hardy Date of the doctoral viva: 28/07/2021 Acknowledgements Many thanks to all the people who supported me on my PhD journey, a life-changing experience. This work was funded by two research grants from the Deutsche Forschungsgemeinschaft (DFG). The related projects were designed as a joint work between two research groups at the University Koblenz-Landau. I am especially grateful to my supervisor Prof. Dr. Sue Williams who provided invaluable support throughout my whole project, particularly through her expertise, as well as her research impulses and discussions. Still, she gave me the freedom to shape my research, so many thanks! Further, I would like to thank my co-advisor Prof. Dr. Petra Schubert for very constructive feedback through the years. I am thankful for having had the unique opportunity to be part of the Center for Enterprise Information Research (CEIR) team with excellent researchers and the IndustryConnect initiative, which helped me bridge the gap between academia and ‘real world’ cases. IndustryConnect, founded by Prof. Dr. Schubert and Prof. Dr. Williams, enabled me to participate in long-term practice-oriented research with industry, a privilege that most other doctoral students cannot enjoy.