Properties of Carbon the Atomic Element Carbon Has Very Diverse
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Package 'Ciaawconsensus'
Package ‘CIAAWconsensus’ September 19, 2018 Type Package Title Isotope Ratio Meta-Analysis Version 1.3 Author Juris Meija and Antonio Possolo Maintainer Juris Meija <[email protected]> Description Calculation of consensus values for atomic weights, isotope amount ratios, and iso- topic abundances with the associated uncertainties using multivariate meta-regression ap- proach for consensus building. License Unlimited LazyData yes Imports mvtnorm, stringr, numDeriv, stats, Matrix NeedsCompilation no Repository CRAN Date/Publication 2018-09-19 13:30:12 UTC R topics documented: abundances2ratios . .2 at.weight . .3 ciaaw.mass.2003 . .4 ciaaw.mass.2012 . .5 ciaaw.mass.2016 . .6 iridium.data . .6 mmm ............................................7 normalize.ratios . .8 platinum.data . .9 Index 10 1 2 abundances2ratios abundances2ratios Isotope ratios of a chemical element from isotopic abundances Description This function calculates the isotope ratios of a chemical element from the given isotopic abundances and their uncertainties. The uncertainty evaluation is done using the propagation of uncertainty and the missing correlations between the isotopic abundances are reconstructed using Monte Carlo methods. Usage abundances2ratios(x, ux, ref=1, iterations=1e4) Arguments x A vector of isotopic abundances of an element ux Standard uncertainties of x ref Index to specify the desired reference isotope for isotope amount ratios iterations Number of iterations for isotopic abundance correlation mapping Details Situations are often encountered where isotopic abundances are reported but not the isotope ratios. In such cases we reconstruct the isotope ratios that are consistent with the abundances and their uncertainties. Given only the abundances and their uncertainties, for elements with four or more isotopes one cannot unambiguously infer the uncertainties of the ratios due to the unknown correla- tions between isotopic abundances. -

This Ubiquitous Carbon…
Engineering Physics Department Presents Dr. Cristian Contescu Senior Research Staff, Materials Science and Technology Division Oak Ridge National Laboratory This ubiquitous carbon… Abstract: After Stone Age, Bronze Age, and Iron Age, and after the Silicon Age of the informational revolution, the technologies of 21st century are marked by the ubiquitous presence of various forms of carbon allotropes. For a very long time, diamond and graphite were the only known carbon allotropes, but that has changed with the serendipitous discovery of fullerenes, carbon nanotubes, and graphene. Every ten or fifteen years scientists unveil new forms of carbons with new and perplexing properties, while computations suggest that the carbon’s family still has members unknown to us today. At a dramatically accelerated pace, new carbon forms find their place at the leading edge of scientific and technological innovations. At the same time traditional forms of carbon are being used in new and exciting applications that make our life safer, healthier, and more enjoyable. The 21st century may soon be recognized as the Age of Carbon forms. This educational talk will show how carbon, the fourth most abundant element in the Galaxy and the basis of life on Earth, was the engine of most important technological developments throughout the history of civilization. It will emphasize the ability of carbon atoms to generate a variety of mutual combinations and with many other chemical elements. These properties have placed carbon at the core of numerous inventions that define our civilization, while emerging new technologies open a rich path for value-added products in today’s market. -

Sp Carbon Chain Interaction with Silver Nanoparticles Probed by Surface Enhanced Raman Scattering
sp carbon chain interaction with silver nanoparticles probed by Surface Enhanced Raman Scattering A. Lucotti1, C. S. Casari2, M. Tommasini1, A. Li Bassi2, D. Fazzi1, V. Russo2, M. Del Zoppo1, C. Castiglioni1, F. Cataldo3, C. E. Bottani2, G. Zerbi1 1 Dipartimento di Chimica, Materiali e Ingegneria Chimica ‘G. Natta’ and NEMAS - Center for NanoEngineered MAterials and Surfaces, Politecnico di Milano, Piazza Leonardo da Vinci 32, I-20133 Milano, Italy 2 Dipartimento di Energia and NEMAS - Center for NanoEngineered MAterials and Surfaces, Politecnico di Milano, via Ponzio 34/3, I-20133 Milano, Italy 3 Actinium Chemical Research srl, via Casilina 1626/A, 00133 Roma, Italy and INAF – Osservatorio Astrofisico di Catania, Via S. Sofia 78, 95123 Catania, Italy Abstract Surface Enhanced Raman Spectroscopy (SERS) is exploited here to investigate the interaction of isolated sp carbon chains (polyynes) in a methanol solution with silver nanoparticles. Hydrogen-terminated polyynes show a strong interaction with silver colloids used as the SERS active medium revealing a chemical SERS effect. SERS spectra after mixing polyynes with silver colloids show a noticeable time evolution. Experimental results, supported by density functional theory (DFT) calculations of the Raman modes, allow us to investigate the behaviour and stability of polyynes of different lengths and the overall sp conversion towards sp2 phase. 1 2 1. Introduction Linear carbon chains with sp hybridization represent one of the simplest one dimensional systems and have therefore attracted a great interest for many years [1, 2]. sp chains can display two types of carbon-carbon bonding: polyynes, chains with single-triple alternating bonds (…-C≡C- C≡C-…) and polycumulenes, chains with all double bonds (…=C=C=C=…). -

Agricultural Soil Carbon Credits: Making Sense of Protocols for Carbon Sequestration and Net Greenhouse Gas Removals
Agricultural Soil Carbon Credits: Making sense of protocols for carbon sequestration and net greenhouse gas removals NATURAL CLIMATE SOLUTIONS About this report This synthesis is for federal and state We contacted each carbon registry and policymakers looking to shape public marketplace to ensure that details investments in climate mitigation presented in this report and through agricultural soil carbon credits, accompanying appendix are accurate. protocol developers, project developers This report does not address carbon and aggregators, buyers of credits and accounting outside of published others interested in learning about the protocols meant to generate verified landscape of soil carbon and net carbon credits. greenhouse gas measurement, reporting While not a focus of the report, we and verification protocols. We use the remain concerned that any end-use of term MRV broadly to encompass the carbon credits as an offset, without range of quantification activities, robust local pollution regulations, will structural considerations and perpetuate the historic and ongoing requirements intended to ensure the negative impacts of carbon trading on integrity of quantified credits. disadvantaged communities and Black, This report is based on careful review Indigenous and other communities of and synthesis of publicly available soil color. Carbon markets have enormous organic carbon MRV protocols published potential to incentivize and reward by nonprofit carbon registries and by climate progress, but markets must be private carbon crediting marketplaces. paired with a strong regulatory backing. Acknowledgements This report was supported through a gift Conservation Cropping Protocol; Miguel to Environmental Defense Fund from the Taboada who provided feedback on the High Meadows Foundation for post- FAO GSOC protocol; Radhika Moolgavkar doctoral fellowships and through the at Nori; Robin Rather, Jim Blackburn, Bezos Earth Fund. -

Introduction to Chemistry
Introduction to Chemistry Author: Tracy Poulsen Digital Proofer Supported by CK-12 Foundation CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook Introduction to Chem... materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based Authored by Tracy Poulsen collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and 8.5" x 11.0" (21.59 x 27.94 cm) distribution of high-quality educational content that will serve both as core text as well as provide Black & White on White paper an adaptive environment for learning. 250 pages ISBN-13: 9781478298601 Copyright © 2010, CK-12 Foundation, www.ck12.org ISBN-10: 147829860X Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made Please carefully review your Digital Proof download for formatting, available to Users in accordance with the Creative Commons Attribution/Non-Commercial/Share grammar, and design issues that may need to be corrected. Alike 3.0 Unported (CC-by-NC-SA) License (http://creativecommons.org/licenses/by-nc- sa/3.0/), as amended and updated by Creative Commons from time to time (the “CC License”), We recommend that you review your book three times, with each time focusing on a different aspect. which is incorporated herein by this reference. Specific details can be found at http://about.ck12.org/terms. Check the format, including headers, footers, page 1 numbers, spacing, table of contents, and index. 2 Review any images or graphics and captions if applicable. -

Glossary of Terms for Carbon Dioxide Capture and Storage
CCS Defined A glossary of terms for Carbon dioxide Capture and Storage Deliverable D.73 of the STEMM-CCS project - 2016 INTRODUCTION: This glossary – ‘CCS Defined’ - has been brought together from many sources, and following comments and advice from co-workers on the Strategies for Environmental Monitoring of Marine Carbon Capture and Storage, STEMM- CCS (654462), CO2 Capture from Cement Production, CEMCAP (641185) and Low Emissions Intensity Lime and Cement, LEILAC (654465) Projects, which form a group under the EC H2020 Carbon Capture and Storage Programme. ‘CCS defined’ (deliverable D.73) comprises an update and broadening of an early glossary (Boot et al, 2013. The Language of CCS) to bring together a comprehensive set of definitions concerned with sub-seabed carbon dioxide capture and storage (CCS) produced as a deliverable of the FP7, ECO2 project (http://www.eco2-project.eu/). The ECO2 “Language of CCS’ was concerned primarily with aspects of sub-seabed storage, ‘CCS Defined’ includes other topics reflecting additional language required by the LEILAC and CEMCAP projects, especially elements of capture technologies, and is widened to include storage in general. It is, therefore, a more complete glossary which should prove useful beyond the immediate projects for which it is written. The aim of producing ‘CCS Defined’ is in the first instance to provide a common vocabulary intended to minimise misunderstandings and confusion across the various scientific disciplines working within CCS. It is NOT intended to provide a document with any legal standing, whatsoever. As with the previous publication, ‘CCS Defined’ has drawn upon a wide range of sources within the relevant literature and across numerous websites so this glossary is very much a compilation of many ideas. -

BLACK CARBON RESEAR RESEARC CH and FUTURE STRATEGIES Reducing Emissions, Improving Hhumanuman Health and Taking Action on Climate Changec Hange
BLACK CARBON RESEAR RESEARC CH AND FUTURE STRATEGIES Reducing emissions, improving hhumanuman health and taking action on climate changec hange Introduction Black carbon is the sooty black material emitted from gas and diesel engines, coal-fired power plants, and other sources that burn fossil fuel. It comprises a significant portion of particulate matter or PM, which is an air pollutant. Black carbon is a global environmental problem that has negative implications for both human health and our climate. Inhalation of black carbon is associated with health problems including respiratory and cardiovascular disease, cancer, and even birth defects. And because of its ability to absorb light as heat, it also contributes to climate change. For example, as black carbon warms the air, rapid changes in patterns of Diesel exhaust black carbon particle (500 nm). Photo byb y NASA. rain and clouds can occur. Nine EPA STAR Research grants, • Measueasurringing black carbon’s mass totaling more than $6.6 million, and if othero ther particles adhere to This absorption quality also impacts were announced in October 2011 to black carbonc arbon polar ice. As black carbon deposits eight universities to research black • Evaluvaluaating ting low-cost and palm- in the Arctic, the particles cover the carbon. The grantees will further sized blackb lack carbon instruments snow and ice, decreasing the Earth’s research the pollutant’s emission that mmaay y give a wider range of ability to reflect the warming rays of sources and its impacts on climate measumeasurrements. ements. the sun, while absorbing heat and change and health. hastening melt. New black carbonc arbon measurement Measurement Research methods hahavvee been tested in This broad and complex role of EPA scientists are working to laboratory aanndd field studies using airborne black carbon is now under improve ways to measure black EPA’s GeoGeosspatialpatial Measurement of intense study by the EPA. -
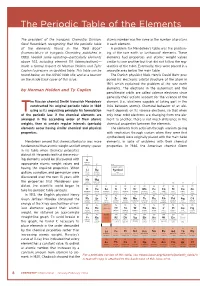
The Periodic Table of the Elements
The Periodic Table of the Elements The president of the Inorganic Chemistry Division, atomic number was the same as the number of protons Gerd Rosenblatt, recognizing that the periodic table in each element. of the elements found in the “Red Book” A problem for Mendeleev’s table was the position- (Nomenclature of Inorganic Chemistry, published in ing of the rare earth or lanthanoid* elements. These 1985) needed some updating—particularly elements elements had properties and atomic weight values above 103, including element 110 (darmstadtium)— similar to one another but that did not follow the reg- made a formal request to Norman Holden and Tyler ularities of the table. Eventually, they were placed in a Coplen to prepare an updated table. This table can be separate area below the main table. found below, on the IUPAC Web site, and as a tear-off The Danish physicist Niels Henrik David Bohr pro- on the inside back cover of this issue. posed his electronic orbital structure of the atom in 1921, which explained the problem of the rare earth by Norman Holden and Ty Coplen elements. The electrons in the outermost and the penultimate orbits are called valence electrons since generally their actions account for the valence of the he Russian chemist Dmitri Ivanovich Mendeleev element (i.e., electrons capable of taking part in the constructed his original periodic table in 1869 links between atoms). Chemical behavior of an ele- Tusing as its organizing principle his formulation ment depends on its valence electrons, so that when of the periodic law: if the chemical elements are only inner orbit electrons are changing from one ele- arranged in the ascending order of their atomic ment to another, there is not much difference in the weights, then at certain regular intervals (periods) chemical properties between the elements. -

Guidelines for the Use of Atomic Weights 5 10 11 12 DOI: ..., Received ...; Accepted
IUPAC Guidelines for the us e of atomic weights For Peer Review Only Journal: Pure and Applied Chemistry Manuscript ID PAC-REC-16-04-01 Manuscript Type: Recommendation Date Submitted by the Author: 01-Apr-2016 Complete List of Authors: van der Veen, Adriaan; VSL Meija, Juris Possolo, Antonio; National Institute of Standards and Technology Hibbert, David; University of New South Wales, School of Chemistry atomic weights, atomic-weight intervals, molecular weight, standard Keywords: atomic weight, measurement uncertainty, uncertainty propagation Author-Supplied Keywords: P.O. 13757, Research Triangle Park, NC (919) 485-8700 Page 1 of 13 IUPAC Pure Appl. Chem. 2016; aop 1 2 3 4 Sponsoring body: IUPAC Inorganic Chemistry Division Committee: see more details on page XXX. 5 IUPAC Recommendation 6 7 Adriaan M. H. van der Veen*, Juris Meija, Antonio Possolo, and D. Brynn Hibbert 8 9 Guidelines for the use of atomic weights 5 10 11 12 DOI: ..., Received ...; accepted ... 13 14 Abstract: Standard atomicFor weights Peer are widely used Review in science, yet the uncertainties Only associated with these 15 values are not well-understood. This recommendation provides guidance on the use of standard atomic 16 weights and their uncertainties. Furthermore, methods are provided for calculating standard uncertainties 17 of molecular weights of substances. Methods are also outlined to compute material-specific atomic weights 10 18 whose associated uncertainty may be smaller than the uncertainty associated with the standard atomic 19 weights. 20 21 Keywords: atomic weights; atomic-weight intervals; molecular weight; standard atomic weight; uncertainty; 22 uncertainty propagation 23 24 25 1 Introduction 15 26 27 Atomic weights provide a practical link the SI base units kilogram and mole. -

Project Note Weston Solutions, Inc
PROJECT NOTE WESTON SOLUTIONS, INC. To: Canadian Radium & Uranium Corp. Site File Date: June 5, 2014 W.O. No.: 20405.012.013.2222.00 From: Denise Breen, Weston Solutions, Inc. Subject: Determination of Significant Lead Concentrations in Sediment Samples References 1. New York State Department of Environmental Conservation. Technical Guidance for Screening Contaminated Sediments. March 1998. [45 pages] 2. U.S. Environmental Protection Agency (EPA) Office of Emergency Response. Establishing an Observed Release – Quick Reference Fact Sheet. Federal Register, Volume 55, No. 241. September 1995. [7 pages] 3. International Union of Pure and Applied Chemistry, Inorganic Chemistry Division Commission on Atomic Weights and Isotopic Abundances. Atomic Weights of Elements: Review 2000. 2003. [120 pages] WESTON personnel collected six sediment samples (including one environmental duplicate sample) from five locations along the surface water pathway of the Canadian Radium & Uranium Corp. (CRU) site in May 2014. The sediment samples were analyzed for Target Analyte List (TAL) Metals and Stable Lead Isotopes. 1. TAL Lead Interpretation: In order to quantify the significance for Lead, Thallium and Mercury the following was performed: 1. WESTON personnel tabulated all available TAL Metal data from the May 2014 Sediment Sampling event. 2. For each analyte of concern (Lead, Thallium, and Mercury), the highest background concentration was selected and then multiplied by three. This is the criteria to find the significance of site attributable release as per Hazard Ranking System guidelines. 3. One analytical lead result (2222-SD04) of 520 mg/kg (J) was qualified with an unknown bias. In accordance with US EPA document “Using Data to Document an Observed Release and Observed Contamination”, 2222-SD03 lead concentration was adjusted by dividing by the factor value for lead of 1.44 to equal 361 mg/kg. -

Carbon-Based Nanomaterials/Allotropes: a Glimpse of Their Synthesis, Properties and Some Applications
materials Review Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications Salisu Nasir 1,2,* ID , Mohd Zobir Hussein 1,* ID , Zulkarnain Zainal 3 and Nor Azah Yusof 3 1 Materials Synthesis and Characterization Laboratory (MSCL), Institute of Advanced Technology (ITMA), Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia 2 Department of Chemistry, Faculty of Science, Federal University Dutse, 7156 Dutse, Jigawa State, Nigeria 3 Department of Chemistry, Faculty of Science, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia; [email protected] (Z.Z.); [email protected] (N.A.Y.) * Correspondence: [email protected] (S.N.); [email protected] (M.Z.H.); Tel.: +60-1-2343-3858 (M.Z.H.) Received: 19 November 2017; Accepted: 3 January 2018; Published: 13 February 2018 Abstract: Carbon in its single entity and various forms has been used in technology and human life for many centuries. Since prehistoric times, carbon-based materials such as graphite, charcoal and carbon black have been used as writing and drawing materials. In the past two and a half decades or so, conjugated carbon nanomaterials, especially carbon nanotubes, fullerenes, activated carbon and graphite have been used as energy materials due to their exclusive properties. Due to their outstanding chemical, mechanical, electrical and thermal properties, carbon nanostructures have recently found application in many diverse areas; including drug delivery, electronics, composite materials, sensors, field emission devices, energy storage and conversion, etc. Following the global energy outlook, it is forecasted that the world energy demand will double by 2050. This calls for a new and efficient means to double the energy supply in order to meet the challenges that forge ahead. -

Glossary of Terms
Glossary of English/Spanish Superfund & WQARF Terms (Note: You may access the bookmark menu at the left to navigate this document more efficiently.) Any ADEQ translation or communication in a language other than English is unofficial and not binding on the State of Arizona. Cualquier traducción o comunicado de ADEQ en un idioma diferente al inglés no es oficial y no sujetará al Estado de Arizona a ninguna obligación jurídica. A Absorption: The passage of one substance into or through another. Absorción: Absorción es el paso de una sustancia a través de otra. Acre-foot: A quantity or volume of water covering one acre to a depth of one foot; equal to 43,560 cubic feet or 325,851 gallons. Acre-pie: Una cantidad o volumen de agua que cubre un acre a una profundidad de un pie; es un equivalente a 43.560 pies cúbicos o 325.851 galones. Activated Carbon: Adsorptive particles or granules of carbon usually obtained by heating carbon (such as wood). These particles or granules have a high capacity to selectively remove certain trace and soluble materials from water. Carbono Activo: Partículas or gránulos de carbono que se obtienen generalmente por medio del calentamiento de carbono (como la madera). Estas partículas o gránulos tienen una alta capacidad de eliminar selectivamente ciertos rastros y materiales solubles del agua. Acute: Occurring over a short period of time; used to describe brief exposures and effects which appear promptly after exposure. Grave: Ocurre durante corto tiempo; se usa para describir breves exposiciones y los efectos que aparecen rapidamente después de una sola exposición.