Package 'Ciaawconsensus'
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Iridium Platinum Alloys
Iridium Platinum Alloys A CRITICAL REVIEW OF THEIR CONSTITUTION AND PROPERTIES By A. s. Darling, Ph.D., A.M.1.Mech.E. Native crystals of iridium-platinum are Journal of Science, in 1860, remarked some- frequently found in association with platinum what incredulously: “The platinum makers of ores. These natural alloys, of rather variable Paris are manufacturing these alloys and composition, occur as grains, and sometimes contrary to the wishes of the discoverers are as small cubes with rounded edges. Although extracting higher prices for them than for the geographical distribution is fairly wide pure platinum”. the major deposits appear to be in the Urals. Prominent physicists were, at this period One such crystal, described in I940 by Masing, of the nineteenth century, devoting consider- Eckhardt and Kloiber (I) had a well-defined able effort to the problems involved in repre- two-phase micro-structure. senting their fundamental and derived units Synthetic alloys were produced at an early in concrete form. Iridium-platinum alloys date. Gaudin (z), who fused spheres of a came to fill a unique position in the scale 10per cent iridium-platinum alloy on hearths of substances capable of retaining indefin- of lime and magnesia, commented in 1838 itely their mass, form, and linear dimensions. upon the lustre, malleability and extreme In addition to their corrosion resistance corrosion resistance of the resultant product. and good mechanical properties they were Twenty years later, however, iridium was readily workable and of fairly high electrical still regarded as a troublesome impurity in resistance. No internal transformations were platinum, but the researches of Sainte-Claire recognised in those days and the alloys were Deville and Debray (3) did much to dispel established as materials par exceZZence for this illusion. -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

Properties of Carbon the Atomic Element Carbon Has Very Diverse
Properties of Carbon The atomic element carbon has very diverse physical and chemical properties due to the nature of its bonding and atomic arrangement. fig. 1 Allotropes of Carbon Some allotropes of carbon: (a) diamond, (b) graphite, (c) lonsdaleite, (d–f) fullerenes (C60, C540, C70), (g) amorphous carbon, and (h) carbon nanotube. Carbon has several allotropes, or different forms in which it can exist. These allotropes include graphite and diamond, whose properties span a range of extremes. Despite carbon's ability to make 4 bonds and its presence in many compounds, it is highly unreactive under normal conditions. Carbon exists in 2 main isotopes: 12C and 13C. There are many other known isotopes, but they tend to be short-lived and have extremely short half-lives. Allotropes The different forms of a chemical element. Cabon is the chemical element with the symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Carbon has 6 protons and 6 Source URL: https://www.boundless.com/chemistry/nonmetallic-elements/carbon/properties-carbon/ Saylor URL: http://www.saylor.org/courses/chem102#6.1 Attributed to: Boundless www.saylor.org Page 1 of 2 neutrons, and has a standard atomic weight of 12.0107 amu. Its electron configuration is denoted as 1s22s22p2. It is a solid, and sublimes at 3,642 °C. It's oxidation state ranges from 4 to -4, and it has an electronegativity rating of 2.55 on the Pauling scale. Carbon has several allotropes, or different forms in which it exists. -

The Separation and Determination of Osmium and Ruthenium
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1969 The epS aration and Determination of Osmium and Ruthenium. Harry Edward Moseley Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Moseley, Harry Edward, "The eS paration and Determination of Osmium and Ruthenium." (1969). LSU Historical Dissertations and Theses. 1559. https://digitalcommons.lsu.edu/gradschool_disstheses/1559 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. ThU dissertation has been 69-17,123 microfilmsd exactly as received MOSELEY, Harry Edward, 1929- THE SEPARATION AND DETERMINATION OF OSMIUM AND RUTHENIUM. Louisiana State University and Agricultural and Mechanical College, PhJ>., 1969 Chemistry, analytical University Microfilms, Inc., Ann Arbor, Michigan THE SEPARATION AND DETERMINATION OF OSMIUM AND RUTHENIUM A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Chemistry Harry Edward Moseley B.S., Lpuisiana State University, 1951 M.S., Louisiana State University, 1952 J anuary, 1969 ACKNOWLEDGMENTS Thanks are due to Dr. Eugene W. Berg under whose direction this work was performed, to Dr. A. D. Shendrikar for his help in the tracer studies, and to Mr. J. H. R. Streiffer for his help in writing the com puter program. -

A History of Iridium OVERCOMING the DIFFICULTIES of MELTING and FABRICATION by L
A History of Iridium OVERCOMING THE DIFFICULTIES OF MELTING AND FABRICATION By L. B. Hunt The Johnson Matthey Group The use in unmanned space craft of iridium to encapsulate the radio- isotope thermoelectric generators, where temperatures of up to 20OO0C have to be withstood over several years of operation, with possible impact velocities of 90 metres per second, has focused greater attention on the remarkable properties of this member of the platinum group of metals. But these same properties of very high melting point and great mechanical strength have been the cause of difficulties in its melting and fabrication over a long period of years. How these problems were tackled and eventually overcome is described in this article. One of the less well-known members of the For some fifty years after the discovery of platinum group, iridium possesses quite platinum in South America and the early remarkable chemical and physical properties. It investigations of its properties by a number of is not only the most resistant of all metals to English, French, German and Swedish scien- corrosion, insoluble in all mineral acids in- tists, it was not realised that the native platinum cluding aqua regia and unattacked by other they were examining also contained other molten metals or by silicates at high tem- elements. The first to recognise that a small in- peratures, but has a very high melting point soluble residue survived the dissolution of and is the only metal to maintain good native platinum in aqua regia was the French mechanical properties in air at temperatures chemist Joseph Louis Proust, working for a above 1600OC.Its great stability can be gauged time in Madrid under the patronage of King from its physical properties, outlined in the Carlos IV, but he failed to grasp that other Table. -

P–V–T Equation of State of Iridium up to 80 Gpa and 3100 K
crystals Article P–V–T Equation of State of Iridium Up to 80 GPa and 3100 K Simone Anzellini 1,* , Leonid Burakovsky 2, Robin Turnbull 3 , Enrico Bandiello 3 and Daniel Errandonea 3 1 Diamond Light Source Ltd., Harwell Science & Innovation Campus, Diamond House, Didcot OX11 0DE, UK 2 Los Alamos National Laboratory, Theoretical Divisions, Los Alamos, NM 87545, USA; [email protected] 3 Departamento de Física Aplicada-Instituto de Ciencia de Materiales, Matter at High Pressure (MALTA) Consolider Team, Universidad de Valencia, Edificio de Investigación, C/Dr. Moliner 50, Burjassot, 46100 Valencia, Spain; [email protected] (R.T.); [email protected] (E.B.); [email protected] (D.E.) * Correspondence: [email protected] Abstract: In the present study, the high-pressure high-temperature equation of the state of iridium has been determined through a combination of in situ synchrotron X-ray diffraction experiments using laser-heating diamond-anvil cells (up to 48 GPa and 3100 K) and density-functional theory calcula- tions (up to 80 GPa and 3000 K). The melting temperature of iridium at 40 GPa was also determined experimentally as being 4260 (200) K. The results obtained with the two different methods are fully consistent and agree with previous thermal expansion studies performed at ambient pressure. The resulting thermal equation of state can be described using a third-order Birch–Murnaghan formalism with a Berman thermal-expansion model. The present equation of the state of iridium can be used as a reliable primary pressure standard for static experiments up to 80 GPa and 3100 K. -

The Radiochemistry of Iridium-- +- -:, G
———..————- !546.8 L472ra NAS-NS 3045 .c.8 THE RADIOCHEMISTRY OF IRIDIUM-- +- -:, G. W. Leddicotte ‘1 Oak Ridge National Laboratory Oak Ridge, Tennessee October 1961 .- .- .. --.—- ---- -. ---- -. —.-. .- ---- ---- --- .- .. .- .— .— –. —-. .— —- -- -—. I -.. - -.. I ..-.- . -. I .. .. .. -- 1.- REPORT selection aids Pinpointing R & D rej9G7t8for hdu.st~ Clearinghouse, Sprhgfleld, Va, 22151 GOVERNMENT-WIDE INDEX—SEMI-MONTHLY GUIDE TO FEDERAL SCIIZNTIYIC AND TECH- NICAL REPORTS. ANNUAL SUBSCRIPTION S22.00 ($27.50 FOREIGN MAILING). SINGLE COPY $3.00. U.S. GOVERNMENT RESEARCH AND DEVELOPMENT REPORTS_EMI-MONTHLY JOURNAL ANNOUNCING R&D REPORTS. ANNUAL SUBSCRIPTION 830.00 ($27A0 FOREIGN MAILING). SINGLE COPY $3.00. FAST ANNOUNCEMENT SERVICE—SUMMARIES OF SELIZCTED R&D REPORTS MAILED ❑ Y INDUSTRIAL CATEGORIES, SS.00 ANNUAL SUBSCRIPTION. WRITE FOR AN APPLICATION FORM. DOCUMENT PRICES~FFECTIVE JANUARY 1, 19s7 THE CLEARINGHOUSE CHANGED ITS PRICING POLICY FOR DOCUMENT SALES FROM A SLIDING PRICE SCALE BASED ON DOCUMENT SIZE TO A SINGLE PRICE FOR DOCUMENTS SOLD. THE NEW DOCUMENT PRICE FOR A PAPER COPY (HARD COPY - HC) DOCUMENT IS $3.00. THE NEW PRICE FOR A MICROFICHE (MFI COPY IS s.ss PER DOcuMENT. THEsE PRICES ALSO ApPLY TO DOCUMENTS ANNOUNCED PRIOR TO JANUARY 1, 1967. THE NEW UNIT PRICES ARE BELOW THE PREVIOUS AVERAGE PRICES OF DOCUMENTS SOLD BY THE CLEARINGHOUSE. EFFICIENCIES IN ORDER PROCESSING RESULTING FROM THE SINGLE PRICE POLICY MAKE THE LOWER AVERAGE PRICE POSSIBLE. THE CLEARINGHOUSE SEL15 COUPONS FOR THE PURCHASE OF DOCUMENTS WHICH SIMPLIFY ORDERING AND HANDLING ❑ Y BOTH THE CLEARINGHOUSE AND ITS CUSTOMERS, PERMITTING THE CLEARINGHOUSE TO GIVE FASTER SERVICE ON DOCUMENT REQUESTS. THE COUPON IS A TABULATING CARD WITH A FACE VALUE OF THE PURCHASE PRICE OF A CLEARINGHOUSE DOCUMENT (S3.00 FOR HC, $.SS FOR MF). -
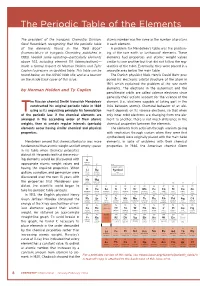
The Periodic Table of the Elements
The Periodic Table of the Elements The president of the Inorganic Chemistry Division, atomic number was the same as the number of protons Gerd Rosenblatt, recognizing that the periodic table in each element. of the elements found in the “Red Book” A problem for Mendeleev’s table was the position- (Nomenclature of Inorganic Chemistry, published in ing of the rare earth or lanthanoid* elements. These 1985) needed some updating—particularly elements elements had properties and atomic weight values above 103, including element 110 (darmstadtium)— similar to one another but that did not follow the reg- made a formal request to Norman Holden and Tyler ularities of the table. Eventually, they were placed in a Coplen to prepare an updated table. This table can be separate area below the main table. found below, on the IUPAC Web site, and as a tear-off The Danish physicist Niels Henrik David Bohr pro- on the inside back cover of this issue. posed his electronic orbital structure of the atom in 1921, which explained the problem of the rare earth by Norman Holden and Ty Coplen elements. The electrons in the outermost and the penultimate orbits are called valence electrons since generally their actions account for the valence of the he Russian chemist Dmitri Ivanovich Mendeleev element (i.e., electrons capable of taking part in the constructed his original periodic table in 1869 links between atoms). Chemical behavior of an ele- Tusing as its organizing principle his formulation ment depends on its valence electrons, so that when of the periodic law: if the chemical elements are only inner orbit electrons are changing from one ele- arranged in the ascending order of their atomic ment to another, there is not much difference in the weights, then at certain regular intervals (periods) chemical properties between the elements. -

Guidelines for the Use of Atomic Weights 5 10 11 12 DOI: ..., Received ...; Accepted
IUPAC Guidelines for the us e of atomic weights For Peer Review Only Journal: Pure and Applied Chemistry Manuscript ID PAC-REC-16-04-01 Manuscript Type: Recommendation Date Submitted by the Author: 01-Apr-2016 Complete List of Authors: van der Veen, Adriaan; VSL Meija, Juris Possolo, Antonio; National Institute of Standards and Technology Hibbert, David; University of New South Wales, School of Chemistry atomic weights, atomic-weight intervals, molecular weight, standard Keywords: atomic weight, measurement uncertainty, uncertainty propagation Author-Supplied Keywords: P.O. 13757, Research Triangle Park, NC (919) 485-8700 Page 1 of 13 IUPAC Pure Appl. Chem. 2016; aop 1 2 3 4 Sponsoring body: IUPAC Inorganic Chemistry Division Committee: see more details on page XXX. 5 IUPAC Recommendation 6 7 Adriaan M. H. van der Veen*, Juris Meija, Antonio Possolo, and D. Brynn Hibbert 8 9 Guidelines for the use of atomic weights 5 10 11 12 DOI: ..., Received ...; accepted ... 13 14 Abstract: Standard atomicFor weights Peer are widely used Review in science, yet the uncertainties Only associated with these 15 values are not well-understood. This recommendation provides guidance on the use of standard atomic 16 weights and their uncertainties. Furthermore, methods are provided for calculating standard uncertainties 17 of molecular weights of substances. Methods are also outlined to compute material-specific atomic weights 10 18 whose associated uncertainty may be smaller than the uncertainty associated with the standard atomic 19 weights. 20 21 Keywords: atomic weights; atomic-weight intervals; molecular weight; standard atomic weight; uncertainty; 22 uncertainty propagation 23 24 25 1 Introduction 15 26 27 Atomic weights provide a practical link the SI base units kilogram and mole. -

Project Note Weston Solutions, Inc
PROJECT NOTE WESTON SOLUTIONS, INC. To: Canadian Radium & Uranium Corp. Site File Date: June 5, 2014 W.O. No.: 20405.012.013.2222.00 From: Denise Breen, Weston Solutions, Inc. Subject: Determination of Significant Lead Concentrations in Sediment Samples References 1. New York State Department of Environmental Conservation. Technical Guidance for Screening Contaminated Sediments. March 1998. [45 pages] 2. U.S. Environmental Protection Agency (EPA) Office of Emergency Response. Establishing an Observed Release – Quick Reference Fact Sheet. Federal Register, Volume 55, No. 241. September 1995. [7 pages] 3. International Union of Pure and Applied Chemistry, Inorganic Chemistry Division Commission on Atomic Weights and Isotopic Abundances. Atomic Weights of Elements: Review 2000. 2003. [120 pages] WESTON personnel collected six sediment samples (including one environmental duplicate sample) from five locations along the surface water pathway of the Canadian Radium & Uranium Corp. (CRU) site in May 2014. The sediment samples were analyzed for Target Analyte List (TAL) Metals and Stable Lead Isotopes. 1. TAL Lead Interpretation: In order to quantify the significance for Lead, Thallium and Mercury the following was performed: 1. WESTON personnel tabulated all available TAL Metal data from the May 2014 Sediment Sampling event. 2. For each analyte of concern (Lead, Thallium, and Mercury), the highest background concentration was selected and then multiplied by three. This is the criteria to find the significance of site attributable release as per Hazard Ranking System guidelines. 3. One analytical lead result (2222-SD04) of 520 mg/kg (J) was qualified with an unknown bias. In accordance with US EPA document “Using Data to Document an Observed Release and Observed Contamination”, 2222-SD03 lead concentration was adjusted by dividing by the factor value for lead of 1.44 to equal 361 mg/kg. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. -

Investigation of the Platinum Metals: IV. Determination of Iridium In
.. INVESTIGATIONS ON THE PLATINUM METALS 1 IV. DETERMINATION OF IRIDIUM IN PLATINUM ALLOYS BY THE METHOD OF FUSION WITH LEAD. By Raleigh Gilchrist. ABSTRACT. A study has been made of the analytical details of the Deville and Stas method for the determination of iridium in platinum alloys containing from o. i to 20 per cent of iridium. Specially prepared alloys made from, highly purified metals were used in the investigation. It was found that the concentration of nitric acid, the concen- tration of aqua regia, the proportion of lead, and the time and the temperature of the lead fusion can be varied over a wide range without affecting the determination. The observations of Deville and Stas that palladium and rhodium have no effect upon the determination and that ruthenium separates quantitatively with the iridium were confirmed. In addition, gold was found not to interfere. Iron sepa- rates nearly quantitatively with the iridium as observed by Deville and Stas. A method for the separation of iron from the iridium was tested and found to give satisfactory results. The loss in weight of crystalline iridium during the ignition periods is insignificant and the weight of crystalline iridium is not affected by heating and cooling in an atmosphere of hydrogen. Spectrographic examination of samples of iridium from analysis showed that neither platinum nor lead was present in sufficient quantities to affect the determination. The iridium results tend to be low by a variable but usually small amount. One factor in this error is a slight solution of iridium by aqua regia. A modified procedure for the method is offered, which combines the optimum conditions for speed and accuracy in the various details of manipulation.