Fed-Std-376B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix A: Symbols and Prefixes
Appendix A: Symbols and Prefixes (Appendix A last revised November 2020) This appendix of the Author's Kit provides recommendations on prefixes, unit symbols and abbreviations, and factors for conversion into units of the International System. Prefixes Recommended prefixes indicating decimal multiples or submultiples of units and their symbols are as follows: Multiple Prefix Abbreviation 1024 yotta Y 1021 zetta Z 1018 exa E 1015 peta P 1012 tera T 109 giga G 106 mega M 103 kilo k 102 hecto h 10 deka da 10-1 deci d 10-2 centi c 10-3 milli m 10-6 micro μ 10-9 nano n 10-12 pico p 10-15 femto f 10-18 atto a 10-21 zepto z 10-24 yocto y Avoid using compound prefixes, such as micromicro for pico and kilomega for giga. The abbreviation of a prefix is considered to be combined with the abbreviation/symbol to which it is directly attached, forming with it a new unit symbol, which can be raised to a positive or negative power and which can be combined with other unit abbreviations/symbols to form abbreviations/symbols for compound units. For example: 1 cm3 = (10-2 m)3 = 10-6 m3 1 μs-1 = (10-6 s)-1 = 106 s-1 1 mm2/s = (10-3 m)2/s = 10-6 m2/s Abbreviations and Symbols Whenever possible, avoid using abbreviations and symbols in paragraph text; however, when it is deemed necessary to use such, define all but the most common at first use. The following is a recommended list of abbreviations/symbols for some important units. -

Foot-Candles: Photometric Units
UPDATED EXTRACT FROM CREG JOURNAL. FILE: FOOT3-UP.DOC REV. 8. LAST SAVED: 01/02/01 15:14 PHOTOMETRICS Foot-Candles: Photometric Units More footnotes on optical topics. David Gibson describes the confusing range of photometric units. A discussion of photometric units may the ratio of luminous efficiency to luminous The non-SI unit mean spherical candle- seem out of place in an electronic journal but efficiency at the wavelength where the eye is power is the intensity of a source if its light engineers frequently have to use light sources most sensitive. Unfortunately, however, this output were spread evenly in all directions. It and detectors. The units of photometry are term can be confused with the term efficacy, is therefore equivalent to the flux [lm] ¸ 4p. some of the most confusing and least which is used to describe the efficiency at standardised of units. converting electrical to luminous power. Luminance Photometric units are not difficult to The candela measures the intensity of a understand, but can be a minefield to the Illumination, Luminous Emittance. point source. We also need to define the uninitiated since many non-SI units are still The illumination of a surface is the properties of an extended source. Each small in use, and there are subtle differences incident power flux density measured in element DS of a diffuse reflective surface will between quantities with similar names, such lumens per square metre. A formal definition scatter the incident flux DF and behave as if as illumination and luminance. would be along the lines of: if a flux DF is it were an infinitesimal point source. -

Pressure, Its Units of Measure and Pressure References
_______________ White Paper Pressure, Its Units of Measure and Pressure References Viatran Phone: 1‐716‐629‐3800 3829 Forest Parkway Fax: 1‐716‐693‐9162 Suite 500 [email protected] Wheatfield, NY 14120 www.viatran.com This technical note is a summary reference on the nature of pressure, some common units of measure and pressure references. Read this and you won’t have to wait for the movie! PRESSURE Gas and liquid molecules are in constant, random motion called “Brownian” motion. The average speed of these molecules increases with increasing temperature. When a gas or liquid molecule collides with a surface, momentum is imparted into the surface. If the molecule is heavy or moving fast, more momentum is imparted. All of the collisions that occur over a given area combine to result in a force. The force per unit area defines the pressure of the gas or liquid. If we add more gas or liquid to a constant volume, then the number of collisions must increase, and therefore pressure must increase. If the gas inside the chamber is heated, the gas molecules will speed up, impact with more momentum and pressure increases. Pressure and temperature therefore are related (see table at right). The lowest pressure possible in nature occurs when there are no molecules at all. At this point, no collisions exist. This condition is known as a pure vacuum, or the absence of all matter. It is also possible to cool a liquid or gas until all molecular motion ceases. This extremely cold temperature is called “absolute zero”, which is -459.4° F. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

SI and CGS Units in Electromagnetism
SI and CGS Units in Electromagnetism Jim Napolitano January 7, 2010 These notes are meant to accompany the course Electromagnetic Theory for the Spring 2010 term at RPI. The course will use CGS units, as does our textbook Classical Electro- dynamics, 2nd Ed. by Hans Ohanian. Up to this point, however, most students have used the International System of Units (SI, also known as MKSA) for mechanics, electricity and magnetism. (I believe it is easy to argue that CGS is more appropriate for teaching elec- tromagnetism to physics students.) These notes are meant to smooth the transition, and to augment the discussion in Appendix 2 of your textbook. The base units1 for mechanics in SI are the meter, kilogram, and second (i.e. \MKS") whereas in CGS they are the centimeter, gram, and second. Conversion between these base units and all the derived units are quite simply given by an appropriate power of 10. For electromagnetism, SI adds a new base unit, the Ampere (\A"). This leads to a world of complications when converting between SI and CGS. Many of these complications are philosophical, but this note does not discuss such issues. For a good, if a bit flippant, on- line discussion see http://info.ee.surrey.ac.uk/Workshop/advice/coils/unit systems/; for a more scholarly article, see \On Electric and Magnetic Units and Dimensions", by R. T. Birge, The American Physics Teacher, 2(1934)41. Electromagnetism: A Preview Electricity and magnetism are not separate phenomena. They are different manifestations of the same phenomenon, called electromagnetism. One requires the application of special relativity to see how electricity and magnetism are united. -

Photography Department Cerritos College
Program Review 2010 Photography Department Cerritos College 1. Description of the Program Mission Statement The mission of the photography department is to educate students in photography by providing a balanced education in both the creative and technical aspects of the photographic medium, the history of photography and the critical analysis of photographs. The mission is accomplished through course offerings that enable students to develop their technical skills, creativity and understanding of the social and historical role and relevance of the photographic medium. a. Course and Program Content i. Course Outlines and Curriculum Development Process The following Photography Department courses have been approved by the curriculum committee: Phot 100 - Introductory Photography (revised and approved 1/25/07) Phot 102 - Intermediate Photography (revised and approved 1/27/05) Phot 110 - Introduction to Digital Photography (revised and approved 3/10/05) Phot 112 - Intermediate Digital Photography (revised and approved 3/10/05) Phot 122 - Color Photography (revised and approved 10/26/06) Phot 130 - Jour 130 - Photojournalism (revised and approved 12/13/07) Phot 150 - Photography and Society (approved 2/26/09) Phot 160 - History of Photography (revised and approved 1/25/07) Phot 171 - Occupational Work Experience in Photography (revised and approved 9/22/05) Phot 200 - Advanced Photography (revised and approved 1/27/05) Phot 230 - Portrait Photography (revised and approved 1/27/05) Phot 232 - Approaches to Studio Lighting (revised and approved 1/27/05) Phot 234 - Portfolio Development (revised and approved 1/26/06) Phot 240 - Experimental Photography (revised and approved 2/24/05) Phot 250 - Contemporary Photographic Expression (revised and approved 12/11/03) Phot 252 - Photography of the Landscape (revised and approved 1/27/05) Phot 298 - Directed Studies Phot 299 - Directed Studies The only course in the list above that has not yet been offered is Photography and Society. -
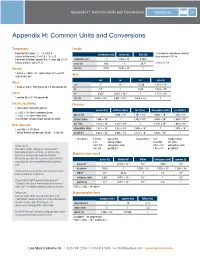
Appendix H: Common Units and Conversions Appendices 215
Appendix H: Common Units and Conversions Appendices 215 Appendix H: Common Units and Conversions Temperature Length Fahrenheit to Celsius: °C = (°F-32)/1.8 1 micrometer (sometimes referred centimeter (cm) meter (m) inch (in) Celsius to Fahrenheit: °F = (1.8 × °C) + 32 to as micron) = 10-6 m Fahrenheit to Kelvin: convert °F to °C, then add 273.15 centimeter (cm) 1 1.000 × 10–2 0.3937 -3 Celsius to Kelvin: add 273.15 meter (m) 100 1 39.37 1 mil = 10 in Volume inch (in) 2.540 2.540 × 10–2 1 –3 3 1 liter (l) = 1.000 × 10 cubic meters (m ) = 61.02 Area cubic inches (in3) cm2 m2 in2 circ mil Mass cm2 1 10–4 0.1550 1.974 × 105 1 kilogram (kg) = 1000 grams (g) = 2.205 pounds (lb) m2 104 1 1550 1.974 × 109 Force in2 6.452 6.452 × 10–4 1 1.273 × 106 1 newton (N) = 0.2248 pounds (lb) circ mil 5.067 × 10–6 5.067 × 10–10 7.854 × 10–7 1 Electric resistivity Pressure 1 micro-ohm-centimeter (µΩ·cm) pascal (Pa) millibar (mbar) torr (Torr) atmosphere (atm) psi (lbf/in2) = 1.000 × 10–6 ohm-centimeter (Ω·cm) –2 –3 –6 –4 = 1.000 × 10–8 ohm-meter (Ω·m) pascal (Pa) 1 1.000 × 10 7.501 × 10 9.868 × 10 1.450 × 10 = 6.015 ohm-circular mil per foot (Ω·circ mil/ft) millibar (mbar) 1.000 × 102 1 7.502 × 10–1 9.868 × 10–4 1.450 × 10–2 2 0 –3 –2 Heat flow rate torr (Torr) 1.333 × 10 1.333 × 10 1 1.316 × 10 1.934 × 10 5 3 2 1 1 watt (W) = 3.413 Btu/h atmosphere (atm) 1.013 × 10 1.013 × 10 7.600 × 10 1 1.470 × 10 1 British thermal unit per hour (Btu/h) = 0.2930 W psi (lbf/in2) 6.897 × 103 6.895 × 101 5.172 × 101 6.850 × 10–2 1 1 torr (Torr) = 133.332 pascal (Pa) 1 pascal (Pa) = 0.01 millibar (mbar) 1.33 millibar (mbar) 0.007501 torr (Torr) –6 A Note on SI 0.001316 atmosphere (atm) 9.87 × 10 atmosphere (atm) 2 –4 2 The values in this catalog are expressed in 0.01934 psi (lbf/in ) 1.45 × 10 psi (lbf/in ) International System of Units, or SI (from the Magnetic induction B French Le Système International d’Unités). -

Light Measurement Handbook from an Oblique Angle, It Should Look As Bright As It Did When Held Perpendicular to Your Line of Vision
Alex Ryer RED Boxes - LINKS to Current Info on Web Site Peabody, MA (USA) To receive International Light's latest Light Measurement Instruments Catalog, contact: InternationalInternational Light Technologies Light 10 Technology Drive Peabody,10 Te11 MA17 01960 Graf Road Tel:New (978) 818-6180 / buryport,Fax: (978) 818-8161 MA 01950 [email protected] / www.intl-lighttech.com Tel: (978) 465-5923 • Fax: (978) 462-0759 [email protected] • http://www.intl-light.com Copyright © 1997 by Alexander D. Ryer All Rights Reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the copyright owner. Requests should be made through the publisher. Technical Publications Dept. International Light Technologies 10 Technology Drive Peabody, MA 01960 ISBN 0-9658356-9-3 Library of Congress Catalog Card Number: 97-93677 Second Printing Printed in the United States of America. 2 Contents 1 What is Light? ........................................................... 5 Electromagnetic Wave Theory........................................... 5 Ultraviolet Light ................................................................. 6 Visible Light ........................................................................ 7 Color Models ....................................................................... 7 Infrared Light ..................................................................... -

The International System of Units (SI)
NAT'L INST. OF STAND & TECH NIST National Institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 330 2001 Edition The International System of Units (SI) 4. Barry N. Taylor, Editor r A o o L57 330 2oOI rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -

Physics, Chapter 30: Magnetic Fields of Currents
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Robert Katz Publications Research Papers in Physics and Astronomy 1-1958 Physics, Chapter 30: Magnetic Fields of Currents Henry Semat City College of New York Robert Katz University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/physicskatz Part of the Physics Commons Semat, Henry and Katz, Robert, "Physics, Chapter 30: Magnetic Fields of Currents" (1958). Robert Katz Publications. 151. https://digitalcommons.unl.edu/physicskatz/151 This Article is brought to you for free and open access by the Research Papers in Physics and Astronomy at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Robert Katz Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 30 Magnetic Fields of Currents 30-1 Magnetic Field around an Electric Current The first evidence for the existence of a magnetic field around an electric current was observed in 1820 by Hans Christian Oersted (1777-1851). He found that a wire carrying current caused a freely pivoted compass needle B D N rDirection I of current D II. • ~ I I In wire ' \ N I I c , I s c (a) (b) Fig. 30-1 Oersted's experiment. Compass needle is deflected toward the west when the wire CD carrying current is placed above it and the direction of the current is toward the north, from C to D. in its vicinity to be deflected. If the current in a long straight wire is directed from C to D, as shown in Figure 30-1, a compass needle below it, whose initial orientation is shown in dotted lines, will have its north pole deflected to the left and its south pole deflected to the right. -
Manual of Style
Manual Of Style 1 MANUAL OF STYLE TABLE OF CONTENTS CHAPTER 1 GENERAL PROVISIONS ...............3 101.0 Scope .............................................. 3 102.0 Codes and Standards..................... 3 103.0 Code Division.................................. 3 104.0 Table of Contents ........................... 4 CHAPTER 2 ADMINISTRATION .........................6 201.0 Administration ................................. 6 202.0 Chapter 2 Definitions ...................... 6 203.0 Referenced Standards Table ......... 6 204.0 Individual Chapter Administrative Text ......................... 6 205.0 Appendices ..................................... 7 206.0 Installation Standards ..................... 7 207.0 Extract Guidelines .......................... 7 208.0 Index ............................................... 9 CHAPTER 3 TECHNICAL STYLE .................... 10 301.0 Technical Style ............................. 10 302.0 Technical Rules ............................ 10 Table 302.3 Possible Unenforceable and Vague Terms ................................ 10 303.0 Health and Safety ........................ 11 304.0 Rules for Mandatory Documents .................................... 11 305.0 Writing Mandatory Requirements ............................... 12 Table 305.0 Typical Mandatory Terms............. 12 CHAPTER 4 EDITORIAL STYLE ..................... 14 401.0 Editorial Style ................................ 14 402.0 Definitions ...................................... 14 403.0 Units of Measure ............................ 15 404.0 Punctuation -

General Constants and Units
Sp.-V/AQuan/1999/10/08:06:45 Page 7 Chapter 2 General Constants and Units Arthur N. Cox 2.1 Mathematical Constants ................. 7 2.2 Physical Constants .................... 8 2.3 General Astronomical Constants ............ 12 2.4 Astronomical Constants Involving Time ....... 13 2.5 Units ............................. 17 2.6 Electric and Magnetic Unit Relations .......... 22 2.1 MATHEMATICAL CONSTANTS [1–3] Constant Number Log π 3.141 592 653 6 0.497 149 872 7 2π 6.283 185 307 2 0.798 179 868 4 4π 12.566 370 614 4 1.099 209 864 0 2 √π 9.869 604 401 1 0.994 299 745 4 π 1.772 453 850 9 0.248 574 936 3 eore 2.718 281 828 5 0.434 294 481 9 mod = M = log e 0.434 294 481 9 0.637 784 311 3 − 1 1/M = ln 10 2.302 585 093 0 0.362 215 688 7 √2 2.000 000 000 0 0.301 029 995 7 √2 1.414 213 562 4 0.150 514 997 8 √ 3 1.732 050 807 6 0.238 560 627 4 10 3.162 277 660 2 0.500 000 000 0 7 Sp.-V/AQuan/1999/10/08:06:45 Page 8 8/2 GENERAL CONSTANTS AND UNITS Constant Number Log ln π 1.144 729 885 8 0.058 703 021 2 eπ 23.140 692 632 8 1.364 376 353 8 Euler constant γ 0.577 215 664 9 0.761 338 108 8 − 1 1 radian rad = 57.◦295 779 513 1 1.758 122 632 4 = 3 437.746 770 78 3.536 273 882 8 = 206 264.806 25 5.314 425 133 2 1◦ = 0.rad017 453 292 5 0.241 877 367 6 − 2 1 = 0.rad000 290 888 2 0.463 726 117 2 − 4 1 = 0.rad000 004 848 1 0.685 574 866 8 − 6 Square degrees on a sphere = 129 600/π = 41 252.961 25.