Medication Code Key: PMCODE and Drug Name in 2007 NHHCS Cdc-Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

No. 33981 2 No
Pretoria, 4 February 2011 Februarle No. 33981 2 No. 33981 GOVERNMENT GAZETTE, 4 FEBRUARY 2011 IMPORTANT NOTICE The Government Printing Works will not be held responsible for faxed documents not received due to errors on the fax machine or faxes received which are unclear or incomplete. Please be advised that an "OK" slip, received from a fax machine, will not be accepted as proof that documents were received by the GPW for printing. If documents are faxed to the GPW it will be the sender's respon sibility to phone and confirm that the documents were received in good order. Furthermore the Government Printing Works will also not be held responsible for cancellations and amendments which have not been done on original documents received from clients. CONTENTS INHOUD Bladsy Koerant Page Gazette No. No. No. No. No. No. GENERAL NOTICE ALGEMENEKENNISGEWING Health, Department of Gesondheld, Departement van General Notice A/gemene Kennisgewing 58 Medicines and Related Substances Act 58 Wet op Beheer van Medisyne en (101/1965): Medicines Control Council: Verwante Stowwe (101/1965): Conditions of registration of a medicine Medisynebeheerraad: Voorwaardes vir in terms of the provisions of section die registrasie van 'n medisyne in terme 15 (7) ..................................................... .. 3 33981 van die bepalings van artikel 15 (7) ........ 4 33981 STAATSKOERANT, 4 FEBRUARIE 2011 No. 33981 3 GENERAL NOTICE ALGEMENE KENNISGEWING NOTICE 58 OF 2011 MEDICINES CONTROL COUNCIL CONDITIONS OF REGISTRATION OF A MEDICINE IN TERMS OF THE PROVISIONS OF SECTION 15(7) OF THE MEDICINES AND RELATED SUBSTANCES ACT, 1965 (ACT 101 OF 1965) 1. The applicant shall ensure that the medicine is manufactured and controlled in terms of the current Good Manufacturing Practices as determined by Council 2. -

Mcneil Consumer : Mdl No
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA IN RE: MCNEIL CONSUMER : MDL NO. 2190 HEALTHCARE, ET AL., MARKETING : AND SALES PRACTICES LITIGATION : : Applies to: : ALL ACTIONS : MEMORANDUM McLaughlin, J. July 13, 2012 This multidistrict litigation arises out of quality control problems at the defendants’ facility manufacturing over- the-counter healthcare products in Fort Washington, Pennsylvania, which led to a series of recalls of those products. The named plaintiffs assert claims for economic loss on behalf of a putative nationwide class against Johnson & Johnson (“J&J”), McNeil Consumer Healthcare (“McNeil”), and four of their executives. The plaintiffs allege that they overpaid for the defendants’ products as a result of the recalls and the defendants’ scheme to conceal or downplay the scope of the quality control problems. The defendants, who have offered a coupon or cash refund to consumers who purchased recalled drugs, have moved to dismiss the operative complaint, and assert that the named plaintiffs lack constitutional standing and have not met the applicable pleading standard. The Court will grant the defendants’ motion because the plaintiffs have not pled facts that show a cognizable injury in fact, which is required to confer Article III standing. I. Procedural Background This litigation resulted from the consolidation of ten individual actions filed around the country. Haviland v. McNeil Consumer Healthcare, No. 10-2195, was filed in this Court on May 12, 2010, asserting economic injuries arising out of the April 30, 2010 recall of over-the-counter children’s drugs by McNeil, a part of the J&J “Family of Companies.” Eight additional cases, also arising out of the April 2010 recall, were filed in district courts around the country.1 All cases asserted claims for economic injury only, with the exception of Rivera v. -

July 21, 2021
1 2nd Quarter 2021 Earnings Call July 21, 2021 Cautionary Note on Forward-looking Statements This presentation contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things: future operating and financial performance, product development, market position and business strategy. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to: risks related to the impact of the COVID-19 global pandemic, such as the scope and duration of the outbreak, government actions and restrictive measures implemented in response, material delays and cancellations of medical procedures, supply chain disruptions and other impacts to the business, or on the Company’s ability to execute business continuity plans, as a result of the COVID-19 pandemic; economic factors, such as interest rate and currency exchange rate fluctuations; competition, including technological advances, new products and patents attained by competitors; challenges inherent in new product research and development, including uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new and existing products; challenges to patents; the impact -

Notice Under S66 of the Commerce Act 1986 Application by Johnson & Johnson to Acquire the Stock, Assets and Business Of
PUBLIC COPY Notice under s66 of the Commerce Act 1986 Application by Johnson & Johnson to acquire the stock, assets and business of the Consumer Healthcare division of Pfizer Inc. COMMERCE ACT 1986: BUSINESS ACQUISITION SECTION 66: NOTICE SEEKING CLEARANCE 28 September 2006 The Registrar Business Acquisitions and Authorisations Commerce Commission PO Box 2351 Wellington Pursuant to s66(1) of the Commerce Act 1986 notice is hereby given seeking clearance of a proposed business acquisition. 518689_1.DOC 2 CONTENTS EXECUTIVE SUMMARY PART 1: TRANSACTION DETAILS 1 The business acquisition for which clearance is sought 2 The person giving this notice 3 Confidentiality 4 Participants 5 Interconnected and associated persons 6 Beneficial interests 7 Links between participants 8 Common directorships 9 Business activities of the participants 10 Reasons for the proposed acquisition PART II: IDENTIFICATION OF MARKETS AFFECTED 11 Horizontal aggregation 12 Differentiated product markets 13 Differentiated product markets 14 Vertical integration 15 Other business acquisitions PARTS III, IV AND V: CONSTRAINTS ON MARKET POWERS BY EXISTING AND POTENTIAL COMPETITION AND OTHER POTENTIAL CONSTRAINTS 16 Allergy medication 17 Products for the treatment of worms 18 Thrush treatment CERTIFICATE APPENDICES 1. Heartburn and indigestion remedies (MYLANTA and MOTILIUM) 2. Worm treatments (COMBANTRIN, VERMOX) 3. Cold, flu, nasal decongestant, cough relief and sort throat medications (CODRAL, SINUTAB, SUDAFED, BENADRYL, BRONDECON) 4. Allergy relief products (ACTIFED, SINUTAB, SUDAFED, VISINE, LIVOSTIN) 518689_1.DOC 3 5. Thrush treatments (DIFLUCAN ONE, DAKTARIN, DAKTAGOLD, NIZORAL, SPORANOX) 6. Shampoo (PREGAINE, ROGAINE, NEUTROGENA, JOHNSON’S BABY SHAMPOO) 7. Hand hygiene (PURELL and MICRO SHIELD) 8. Competitor worm treatment products 9. Multinational pharmaceutical businesses: GlaxoSmithKline, Douglas Pharmaceuticals, Alphapharm, Bayer Group 10. -

Advanced Textiles for Wound Care
Woodhead Publishing in Textiles: Number 85 Advanced textiles for wound care Edited by S. Rajendran Oxford Cambridge New Delhi © 2009 Woodhead Publishing Limited The Textile Institute and Woodhead Publishing The Textile Institute is a unique organisation in textiles, clothing and footwear. Incorporated in England by a Royal Charter granted in 1925, the Institute has individual and corporate members in over 90 countries. The aim of the Institute is to facilitate learning, recognise achievement, reward excellence and disseminate information within the global textiles, clothing and footwear industries. Historically, The Textile Institute has published books of interest to its members and the textile industry. To maintain this policy, the Institute has entered into partnership with Woodhead Publishing Limited to ensure that Institute members and the textile industry continue to have access to high calibre titles on textile science and technology. Most Woodhead titles on textiles are now published in collaboration with The Textile Institute. Through this arrangement, the Institute provides an Editorial Board which advises Woodhead on appropriate titles for future publication and suggests possible editors and authors for these books. Each book published under this arrangement carries the Institute’s logo. Woodhead books published in collaboration with The Textile Institute are offered to Textile Institute members at a substantial discount. These books, together with those published by The Textile Institute that are still in print, are offered on the Woodhead web site at: www.woodheadpublishing.com. Textile Institute books still in print are also available directly from the Institute’s website at: www.textileinstitutebooks.com. A list of Woodhead books on textile science and technology, most of which have been published in collaboration with The Textile Institute, can be found at the end of the contents pages. -
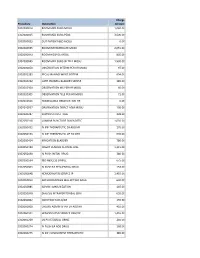
Procedure Description Charge Amount 0302000014 ROOM MED
Charge Procedure Description Amount 0302000014 ROOM MED SURG MOSU 1,040.00 0302000015 ROOM MED SURG PEDS 3,500.00 0302000033 OUT PATIENT BED MOSU 0.00 0302000035 ROOM INTERMEDIATE MOSU 2,055.00 0302000043 ROOM HOSPICE MOSU 805.00 0302000045 ROOM MED SURG W TELE MOSU 1,530.00 0302000050 OBSERVATION INTERM PER HR MOSU 97.00 0302002283 FECAL MANAGEMENT SYSTEM 694.00 0302010232 CATH INDWELL BLADDER SIMPLE 185.00 0302010304 OBSERVATION MS PER HR MOSU 60.00 0302010305 OBSERVATION TELE PER HR MOSU 71.00 0302010556 NONBILLABLE OBSERVATION HR 0.00 0302010557 OBSERVATION DIRECT ADM MOSU 130.00 0302020287 SUPPLIES CHEST TUBE 228.00 0302050318 LUMBAR PUNCTURE DIAGNOSTIC 1,035.00 0302050451 IV INF THERAPEUTIC EA ADD HR 170.00 0302050453 IV INF THERAPEUTIC UP TO 1HR 635.00 0302050454 IRRIGATION BLADDER 780.00 0302050480 INSERT VENOUS CENTRAL LINE 1,315.00 0302050490 IV PUSH INITIAL DRUG 380.00 0302050534 I&D ABSCESS SIMPLE 675.00 0302050603 IV PUSH EA SEQUENTIAL DRUG 153.00 0302050648 HEMODIALYSIS SERVICE IP 1,455.00 0302050813 ARTHROCENTESIS MAJ JNT WO IMAG 630.00 0302050885 ADMIN IMMUNIZATION 145.00 0302050948 DIALYSIS INTRAPERITONEAL SERV 655.00 0302060002 INJECTION SUB-Q/IM 155.00 0302060008 CHEMO ADMIN IV INF EA ADD HR 410.00 0302060101 HEMODIALYSIS SERVICE OBS/OP 1,455.00 0302060269 US PV RESIDUAL URINE 240.00 0302060274 IV PUSH EA ADD DRUG 168.00 0302060275 IV INF CONCURRENT THERAPEUTIC 385.00 0302060276 IV INF SEQUENTIAL THER UP TO 1 191.00 0302060293 INSERT STRAIGHT CATH THERAPEUT 185.00 0302060372 CHEMO ADMIN IV INF SEQ 1 HR 525.00 0302060373 -

Cosmetic Formulation of Skin Care Products.Pdf
DK9685_half-series-title 4/25/06 4:34 PM Page A Cosmetic Formulation of Skin Care Products DK9685_half-series-title 4/25/06 4:34 PM Page B COSMETIC SCIENCE AND TECHNOLOGY Series Editor ERIC JUNGERMANN Jungermann Associates, Inc. Phoenix, Arizona 1. Cosmetic and Drug Preservation: Principles and Practice, edited by Jon J. Kabara 2. The Cosmetic Industry: Scientific and Regulatory Foundations, edited by Norman F. Estrin 3. Cosmetic Product Testing: A Modern Psychophysical Approach, Howard R. Moskowitz 4. Cosmetic Analysis: Selective Methods and Techniques, edited by P. Boré 5. Cosmetic Safety: A Primer for Cosmetic Scientists, edited by James H. Whittam 6. Oral Hygiene Products and Practice, Morton Pader 7. Antiperspirants and Deodorants, edited by Karl Laden and Carl B. Felger 8. Clinical Safety and Efficacy Testing of Cosmetics, edited by William C. Waggoner 9. Methods for Cutaneous Investigation, edited by Robert L. Rietschel and Thomas S. Spencer 10. Sunscreens: Development, Evaluation, and Regulatory Aspects, edited by Nicholas J. Lowe and Nadim A. Shaath 11. Glycerine: A Key Cosmetic Ingredient, edited by Eric Jungermann and Norman O. V. Sonntag 12. Handbook of Cosmetic Microbiology, Donald S. Orth 13. Rheological Properties of Cosmetics and Toiletries, edited by Dennis Laba 14. Consumer Testing and Evaluation of Personal Care Products, Howard R. Moskowitz 15. Sunscreens: Development, Evaluation, and Regulatory Aspects. Second Edition, Revised and Expanded, edited by Nicholas J. Lowe, Nadim A. Shaath, and Madhu A. Pathak DK9685_half-series-title 4/25/06 4:34 PM Page C 16. Preservative-Free and Self-Preserving Cosmetics and Drugs: Principles and Practice, edited by Jon J. -

TITLE Safety and Efficacy of Over-The-Counter Drug Use by the Elderly
" DOCUMENT RESUME ED 245 148 CG 017 518 TITLE Safety and Efficacy of Over-the-Counter Drug Use by the Elderly. Hearing before the Subcommittee on Health and Long-Term Care of the Select Committee on Aging. House of Representatives, Ninety-Eighth Congress, First Session. INSTITUTION Congress of the U.S., Washington, D.C. House Select Committee on Aging. REPORT NO House-Comm-Pub-98-409 PUB DATE 21 Jul 83 NOTE 507p.; Some pages are marginally legible due to small print. PUB TYPE Legal/Legislative/Regulatory Materials (090) EDRS PRICE MF02 Plus Postage. PC Not Available from EDRS. DESCRIPTORS *Consumer Protection; Dietetics; Drug Legislation; *Drug Use; Gerontology; Hearings; *Older Adults; Physical Health; *Safety IDENTIFIERS Congress 98th; Long Term Care; *Nonprescription Drugs; *PPA ABSTRACT This document contains the prepared statements and panel testimony from the Congressional hearing on over-the-counter (OTC) drug use by the elderly. Opening statements are given by Representatives Claude Pepper (chairman), Ralph Regula, Mary Rose Oakar, Michael Bilirakis, Tom Lantos, and Hal Daub. Topics which are covered include the incidence and quantity of drug use by the elderly, health risks, adverse reactions, phenylpropanolamine (PPA), consumer protection, and the Federal Drug Administration's (FDA) role in the OTC drug safety and reguIatiem. Testimony of the first panel on OTC drugs, particularly weight reduction medications containing PPA, is given by representatives of the Health Research Group, the National Broadcasting Company, and consumers. Testimony of the second panel on mail fraud schemes perpetrated against senior citizens is given by consumer advocates representing the United States Postal Service, Criminal Investigations and Consumer Protection Divisions, and the Center for Science in the Public Interest. -

Debridement of Chronic Wounds: a Systematic Review
Health Technology Assessment 1999; Vol. 3: No. 17 (Pt 1) Review The debridement of chronic wounds: a systematic review M Bradley N Cullum T Sheldon Health Technology Assessment NHS R&D HTA Programme HTA HTA How to obtain copies of this and other HTA Programme reports. An electronic version of this publication, in Adobe Acrobat format, is available for downloading free of charge for personal use from the HTA website (http://www.hta.ac.uk). A fully searchable CD-ROM is also available (see below). Printed copies of HTA monographs cost £20 each (post and packing free in the UK) to both public and private sector purchasers from our Despatch Agents. Non-UK purchasers will have to pay a small fee for post and packing. For European countries the cost is £2 per monograph and for the rest of the world £3 per monograph. You can order HTA monographs from our Despatch Agents: – fax (with credit card or official purchase order) – post (with credit card or official purchase order or cheque) – phone during office hours (credit card only). Additionally the HTA website allows you either to pay securely by credit card or to print out your order and then post or fax it. Contact details are as follows: HTA Despatch Email: [email protected] c/o Direct Mail Works Ltd Tel: 02392 492 000 4 Oakwood Business Centre Fax: 02392 478 555 Downley, HAVANT PO9 2NP, UK Fax from outside the UK: +44 2392 478 555 NHS libraries can subscribe free of charge. Public libraries can subscribe at a very reduced cost of £100 for each volume (normally comprising 30–40 titles). -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Propylene Glycol
PROPYLENE GLYCOL Your patch test result indicates that you have a contact allergy to propylene glycol. This contact allergy may cause your skin to react when it is exposed to this substance although it may take several days for the symptoms to appear. Typical symptoms include redness, swelling, itching, and fluid-filled blisters. Where is propylene glycol found? Propylene glycol is used as a softening agent, preservative, humectants, and solvent in cosmetics, fragrances, topical medications, soaps and cleansers, hair care products, and deodorants. Propylene glycol is also found in oral treatments as well as many foods. It is also added during the manufacture of many industrial fluids, such as solvents, thinners, antifreeze, other de-icing fluids, desiccants, brake fluids, and polyester resins. How can you avoid contact with propylene glycol? Avoid products that list any of the following names in the ingredients: • Propylene glycol • 1,2-Dihydroxypropane • CASRN: 57-55-6 • Methylethyl glycol • 1,2-Propanediol • 2-Hydroxypropanol • Isopropylene glycol What are some products that may contain propylene glycol? Antiperspirants and Deodorants: • Old Spice High Endurance • Meguiars Vinyl/Rubber Cleaner/Condition • Adidas 24 Hour Deodorant Control Antiperspirant & Deodorant • Pennzoil Roadside Fix A Flat Tire Sealant & • Adidas 24 Hour Fragrance Clear Stick • Old Spice High Endurance Deodorant Flat Preventative Deodorant • Old Spice Red Zone Clear Gel • Rain-X De-Icer (Aerosol) • Adidas Action 3 Tech F • Old Spice Red Zone Deodorant Stick • Slime -

Pg 1 of 189 Products and Pricing on the Alberta Blue Cross Drug Price List (ABCDPL) Effective April 13, 2017
Products and Pricing on the Alberta Blue Cross Drug Price List (ABCDPL) Effective April 13, 2017 DIN/ PIN/ NPN Product Description Route Form MFR Unit of Issue Base Price 00002192691 3TC 10 MG/ML ORAL LIQUID ORL LIQ GSK ML 0.3307 00002192683 3TC 150 MG TABLET ORL TAB GSK TAB 5.0507 00002247825 3TC 300 MG TABLET ORL TAB GSK TAB 9.8070 00002162113 5 BENZAGEL 5% TOPICAL (ALCOHOL) GEL TOP ALCGEL CLC G 0.2080 00002166607 5 BENZAGEL 5% TOPICAL LOTION TOP LOT NVC ML 0.1984 00000437999 5% DEXTROSE & 0.45% NACL W 0.15% KCL 20 MEQ INJ INJ DEFAUL BAX ML 0.0030 00000037974 50% DEXTROSE 500 MG/ML INJECTION USP INJ DEFAUL HSP ML 0.5842 00080013172 5-HTP 100 MG CAPSULE ORL CAP VTH CAP 0.2000 00002414570 ABBOTT-CITALOPRAM 10 MG TABLET ORL TAB ABB TAB 0.1432 00002414589 ABBOTT-CITALOPRAM 20 MG TABLET ORL TAB ABB TAB 0.2397 00002414597 ABBOTT-CITALOPRAM 40 MG TABLET ORL TAB ABB TAB 0.2397 00002412942 ABBOTT-CLOPIDOGREL 75 MG TABLET ORL TAB ABB TAB 0.4735 00002414805 ABBOTT-LEVETIRACETAM 250 MG TABLET ORL TAB ABB TAB 0.8000 00002414791 ABBOTT-LEVETIRACETAM 500 MG TABLET ORL TAB ABB TAB 0.9750 00002414783 ABBOTT-LEVETIRACETAM 750 MG TABLET ORL TAB ABB TAB 1.3500 00002414546 ABBOTT-OLANZAPINE ODT 10 MG TABLET ORL DISNTAB ABB TAB 1.2857 00002414538 ABBOTT-OLANZAPINE ODT 5 MG TABLET ORL DISNTAB ABB TAB 0.6434 00002412969 ABBOTT-PANTOPRAZOLE 40 MG ENTERIC-COATED TABLET ORL ECT ABB TAB 0.3628 00002412985 ABBOTT-QUETIAPINE 100 MG TABLET ORL TAB ABB TAB 0.3295 00002412993 ABBOTT-QUETIAPINE 200 MG TABLET ORL TAB ABB TAB 0.6617 00002412977 ABBOTT-QUETIAPINE 25 MG TABLET ORL TAB ABB TAB 0.1235 00002413000 ABBOTT-QUETIAPINE 300 MG TABLET ORL TAB ABB TAB 0.9656 00002422638 ABBOTT-RABEPRAZOLE 10 MG ENTERIC-COATED TABLET ORL ECT ABB TAB 0.1204 00002422646 ABBOTT-RABEPRAZOLE 20 MG ENTERIC-COATED TABLET ORL ECT ABB TAB 0.2408 00002414619 ABBOTT-TOPIRAMATE 100 MG TABLET ORL TAB ABB TAB 0.5928 This document is for pricing use only and should not be used to determine benefit status information.