Notice Under S66 of the Commerce Act 1986 Application by Johnson & Johnson to Acquire the Stock, Assets and Business Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Faqs on COVID-19 Vaccine
COVID-19 Frequently Asked Questions COVID-19 Vaccines About COVID-19 Vaccines Q: Why is a COVID-19 vaccine needed if social distancing and wearing masks prevent the COVID-19 virus from spreading? A: Vaccines boost your immune system, so it will be ready to fight the virus if you are exposed. Vaccination combined with ongoing prevention efforts including wearing face masks that cover the mouth and nose, frequent hand washing and staying at least 6 feet away from others offer the best protection against COVID-19. Q: How many COVID-19 vaccines are available? Which one should I get? A: In the United States, three COVID-19 vaccines have been granted emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA) and recommended for use by the Centers for Disease Control and Prevention (CDC). These vaccines, manufactured by Pfizer, Moderna, and Johnson & Johnson (Janssen), have all been proven safe and effective at preventing serious illness, hospitalization, and death from COVID-19 disease. The CDC recommends getting the first vaccine available to you for protection from COVID-19. While vaccine supply is limited, individuals likely will not get to choose which vaccine to receive, and will be given what is available from their vaccine provider at the time they receive their immunization. Q: Are all of the COVID-19 vaccines effective? A: Yes. COVID-19 vaccines from Pfizer, Moderna and Johnson & Johnson (Janssen) have been approved for emergency use by the FDA, and recommended for use by the CDC after a rigorous analysis proved their effectiveness. During studies, all the vaccines were shown to prevent serious illness from COVID-19 at high effectiveness rates. -

2015 Annual Report
ANNUAL REPORT 2015 MARCH 2016 TO OUR SHAREHOLDERS ALEX GORSKY Chairman, Board of Directors and Chief Executive Officer This year at Johnson & Johnson, we are proud this aligned with our values. Our Board of WRITTEN OVER to celebrate 130 years of helping people Directors engages in a formal review of 70 YEARS AGO, everywhere live longer, healthier and happier our strategic plans, and provides regular OUR CREDO lives. As I reflect on our heritage and consider guidance to ensure our strategy will continue UNITES & our future, I am optimistic and confident in the creating better outcomes for the patients INSPIRES THE long-term potential for our business. and customers we serve, while also creating EMPLOYEES long-term value for our shareholders. OF JOHNSON We manage our business using a strategic & JOHNSON. framework that begins with Our Credo. Written OUR STRATEGIES ARE BASED ON over 70 years ago, it unites and inspires the OUR BROAD AND DEEP KNOWLEDGE employees of Johnson & Johnson. It reminds OF THE HEALTH CARE LANDSCAPE us that our first responsibility is to the patients, IN WHICH WE OPERATE. customers and health care professionals who For 130 years, our company has been use our products, and it compels us to deliver driving breakthrough innovation in health on our responsibilities to our employees, care – from revolutionizing wound care in communities and shareholders. the 1880s to developing cures, vaccines and treatments for some of today’s most Our strategic framework positions us well pressing diseases in the world. We are acutely to continue our leadership in the markets in aware of the need to evaluate our business which we compete through a set of strategic against the changing health care environment principles: we are broadly based in human and to challenge ourselves based on the health care, our focus is on managing for the results we deliver. -

No. 33981 2 No
Pretoria, 4 February 2011 Februarle No. 33981 2 No. 33981 GOVERNMENT GAZETTE, 4 FEBRUARY 2011 IMPORTANT NOTICE The Government Printing Works will not be held responsible for faxed documents not received due to errors on the fax machine or faxes received which are unclear or incomplete. Please be advised that an "OK" slip, received from a fax machine, will not be accepted as proof that documents were received by the GPW for printing. If documents are faxed to the GPW it will be the sender's respon sibility to phone and confirm that the documents were received in good order. Furthermore the Government Printing Works will also not be held responsible for cancellations and amendments which have not been done on original documents received from clients. CONTENTS INHOUD Bladsy Koerant Page Gazette No. No. No. No. No. No. GENERAL NOTICE ALGEMENEKENNISGEWING Health, Department of Gesondheld, Departement van General Notice A/gemene Kennisgewing 58 Medicines and Related Substances Act 58 Wet op Beheer van Medisyne en (101/1965): Medicines Control Council: Verwante Stowwe (101/1965): Conditions of registration of a medicine Medisynebeheerraad: Voorwaardes vir in terms of the provisions of section die registrasie van 'n medisyne in terme 15 (7) ..................................................... .. 3 33981 van die bepalings van artikel 15 (7) ........ 4 33981 STAATSKOERANT, 4 FEBRUARIE 2011 No. 33981 3 GENERAL NOTICE ALGEMENE KENNISGEWING NOTICE 58 OF 2011 MEDICINES CONTROL COUNCIL CONDITIONS OF REGISTRATION OF A MEDICINE IN TERMS OF THE PROVISIONS OF SECTION 15(7) OF THE MEDICINES AND RELATED SUBSTANCES ACT, 1965 (ACT 101 OF 1965) 1. The applicant shall ensure that the medicine is manufactured and controlled in terms of the current Good Manufacturing Practices as determined by Council 2. -

Mcneil Consumer : Mdl No
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA IN RE: MCNEIL CONSUMER : MDL NO. 2190 HEALTHCARE, ET AL., MARKETING : AND SALES PRACTICES LITIGATION : : Applies to: : ALL ACTIONS : MEMORANDUM McLaughlin, J. July 13, 2012 This multidistrict litigation arises out of quality control problems at the defendants’ facility manufacturing over- the-counter healthcare products in Fort Washington, Pennsylvania, which led to a series of recalls of those products. The named plaintiffs assert claims for economic loss on behalf of a putative nationwide class against Johnson & Johnson (“J&J”), McNeil Consumer Healthcare (“McNeil”), and four of their executives. The plaintiffs allege that they overpaid for the defendants’ products as a result of the recalls and the defendants’ scheme to conceal or downplay the scope of the quality control problems. The defendants, who have offered a coupon or cash refund to consumers who purchased recalled drugs, have moved to dismiss the operative complaint, and assert that the named plaintiffs lack constitutional standing and have not met the applicable pleading standard. The Court will grant the defendants’ motion because the plaintiffs have not pled facts that show a cognizable injury in fact, which is required to confer Article III standing. I. Procedural Background This litigation resulted from the consolidation of ten individual actions filed around the country. Haviland v. McNeil Consumer Healthcare, No. 10-2195, was filed in this Court on May 12, 2010, asserting economic injuries arising out of the April 30, 2010 recall of over-the-counter children’s drugs by McNeil, a part of the J&J “Family of Companies.” Eight additional cases, also arising out of the April 2010 recall, were filed in district courts around the country.1 All cases asserted claims for economic injury only, with the exception of Rivera v. -

IN the UNITED STATES DISTRICT COURT for the DISTRICT of DELAWARE ALZA CORPORATION and JANSSEN PHARMACEUTICALS, INC., Plaintiffs
Case 1:16-cv-00914-UNA Document 3 Filed 10/07/16 Page 1 of 20 PageID #: 24 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE ALZA CORPORATION and ) JANSSEN PHARMACEUTICALS, INC., ) ) Plaintiffs, ) ) v. ) Civil Action No. ____________ ) REDACTED PUBLIC VERSION AMNEAL PHARMACEUTICALS OF ) NEW YORK, LLC and ) AMNEAL PHARMACEUTICALS LLC, ) ) Defendants. ) COMPLAINT In this patent infringement action, Plaintiffs ALZA Corporation ("ALZA") and Janssen Pharmaceuticals, Inc. (collectively "Plaintiffs"), for their complaint against Defendants Amneal Pharmaceuticals of New York, LLC ("Amneal Pharms. NY") and Amneal Pharmaceuticals LLC ("Amneal Pharms. LLC") (collectively, "Amneal"), allege as follows: NATURE OF THE ACTION 1. This is an action for patent infringement under the patent laws of the United States, Title 35, United States Code, in response to, inter alia, the submission by Amneal of Abbreviated New Drug Application ("ANDA") No. 207515, with the U.S. Food and Drug Administration ("FDA") seeking approval to manufacture and sell a generic version of CONCERTA® prior to the expiration of U.S. Patent No. 8,163,798 ("the '798 patent") and U.S. Patent No. 9,144,549 ("the '549 patent"). PARTIES 2. Plaintiff ALZA is a Delaware corporation, having its principal place of business at 700 Eubanks Drive, Vacaville, California 95688. Case 1:16-cv-00914-UNA Document 3 Filed 10/07/16 Page 2 of 20 PageID #: 25 REDACTED PUBLIC VERSION 3. Plaintiff Janssen Pharmaceuticals, Inc. ("Janssen") is a Pennsylvania corporation, having a place of business at 1125 Trenton-Harbourton Road, Titusville, New Jersey 08560. 4. On information and belief, Defendant Amneal Pharms. LLC is a limited liability company organized under the laws of the State of Delaware and has a place of business at 400 Crossing Boulevard, Bridgewater, NJ 08807. -

Research & Development of Vaccines to Prevent SARS-Cov2 Infection
Monthly update Research & development of vaccines to prevent SARS-coV2 infection Updated May 2021 Disclaimer No vaccine against COVID-19 is approved. This document does not provide guidance on what vaccine or medicines to take. Please avoid self-prescription and always refer to your doctor before making any treatment decision. This document provides a selection of updates on the research and development of vaccines for the current coronavirus infection. Those highlights are for the information of patient organisations/ groups, advocates and people living with a rare disease. EURORDIS takes reasonable steps to verify the accuracy of the information presented. This document does not constitute, and shall not be deemed or construed as, any approval or endorsement by EURORDIS of such product or entity. Updated May 2021 Contents (click to navigate in document) A ‘must-read’ introduction .................................................................................................................................................................................................................................. 4 Resources ........................................................................................................................................................................................................................................................... 5 Vaccines in development ................................................................................................................................................................................................................................... -

Medication Other Information Aches and Pains Constipation Cough/Cold Diarrhea Fever Over the Counter Medications in Pregnancy
Over the Counter Medications in Pregnancy Women commonly use over the counter medications in pregnancy. This is a list of those medication you may safely use during pregnancy. If you have any questions about these medications and how to use them please contact your Healthcare Provider's office. Unless otherwise stated please take the medication as directed on the manufactures label. Medication Other Information Aches and Pains * Tylenol Extra Strength 500 mg tablet No more than 6 tablets in a 24 hour period * Tylenol 325 mg tablet No more than 8 in a 24 hour period Constipation Stool Softener Increases the amount of water in your stools to make them easier to pass * Colace (docusate sodium) * Surfak (docusate calcium) * Docusate Fiber Laxative Increases the amount of bulk in your stools to make them easier to pass * Metamucil (psyllium) * Fibercon (Calcium polycarbophil 625 mg) Stool Softener/Fiber Laxative Increases the amount of water in your stools to make them easier to pass * Peri-Colace (docusate sodium/sennosides) * Senekot -S (docusate sodium/sennosides) Osmotic Laxative Increases the amount of water in your stools to make them easier to pass * Milk of Magnesia * MiraLax (polyethylene glycol 3350) Cough/Cold Expectorants Help thin mucus and phlegm so they can be coughed up * Robitussin * Guaifenesin Antihistamines Can be used to relieve seasonal allergy and common cold symptoms of nasal congestion, * Chlorpheniramine sneezing and itchy eyes Cough Suppressants Help calm a cough * Dextromethorphan Decongestants Are used to relieve -

Management Team
Management Team Bruce C. Cozadd Executive Chairman Bruce Cozadd joined Jazz Pharmaceuticals at its inception. From 2001 until he joined Jazz Pharmaceuticals, Mr. Cozadd served as a consultant to companies in the biopharmaceutical industry. From 1991 until 2001, he held various positions with ALZA Corporation, a pharmaceutical company now owned by Johnson & Johnson, most recently as its Executive Vice President and Chief Operating Officer, with responsibility for research and development, manufacturing and sales and marketing. Previously at ALZA Corporation he held the roles of Chief Financial Officer and Vice President, Corporate Planning and Analysis. Mr. Cozadd received a B.S. from Yale University and an M.B.A. from the Stanford Graduate School of Business. Mr. Cozadd serves on the boards of Cerus Corporation, a biopharmaceutical company; Threshold Pharmaceuticals, a biotechnology company; and The Nueva School and Stanford Hospital and Clinics, both non-profit organizations. Samuel R. Saks, MD Chief Executive Officer Samuel Saks, M.D., joined Jazz Pharmaceuticals at its inception. From 2001 until he joined Jazz Pharmaceuticals, Dr. Saks was Company Group Chairman of ALZA Corporation and served as a member of the Johnson & Johnson Pharmaceutical Group Operating Committee. From 1992 until 2001, he held various positions with ALZA Corporation, most recently as its Chief Medical Officer and Group Vice President, where he was responsible for clinical and commercial activities. Dr. Saks received a B.S. and an M.D. from the University of Illinois. Dr. Saks serves on the board of Trubion Pharmaceuticals and Cougar Biotechnology. Robert M. Myers President Robert Myers joined Jazz Pharmaceuticals at its inception and was appointed as Jazz Pharmaceuticals’ President in March 2007. -
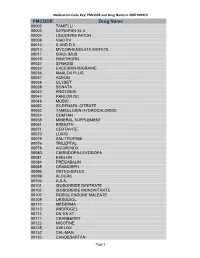
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS Cdc-Pdf
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00002 TAMIFLU 00003 DITROPAN XL II 00004 LIDODERM PATCH 00008 VIACTIV 00010 A AND D II 00013 MYCOPHENOLATE MOFETIL 00017 SIROLIMUS 00019 HAWTHORN 00027 SYNAGIS 00032 EXCEDRIN MIGRAINE 00036 MAALOX PLUS 00037 ACEON 00038 GLYSET 00039 SONATA 00042 PROTONIX 00044 PANLOR DC 00048 MOBIC 00052 SILDENAFIL CITRATE 00053 TAMSULOSIN HYDROCHLORIDE 00054 COMTAN 00058 MINERAL SUPPLEMENT 00061 BISMUTH 00071 CERTAVITE 00073 LUXIQ 00075 SAL-TROPINE 00076 TRILEPTAL 00078 AGGRENOX 00080 CARBIDOPA-LEVODOPA 00081 EXELON 00084 PREGABALIN 00085 ORAMORPH 00096 OSTEO-BIFLEX 00099 ALOCRIL 00100 A.S.A. 00101 ISOSORBIDE DINITRATE 00102 ISOSORBIDE MONONITRATE 00107 ROSIGLITAZONE MALEATE 00109 URSODIOL 00112 MEDERMA 00113 ANDROGEL 00114 DILTIA XT 00117 CRANBERRY 00123 NICOTINE 00125 AVELOX 00132 CAL-MAG 00133 CANDESARTAN Page 1 Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00148 PROLIXIN D 00149 D51/2 NS 00150 NICODERM CQ PATCH 00151 TUSSIN 00152 CEREZYME 00154 CHILDREN'S IBUPROFEN 00156 PROPOXACET-N 00159 KALETRA 00161 BISOPROLOL 00167 NOVOLIN N 00169 KETOROLAC TROMETHAMINE 00172 OPHTHALMIC OINTMENT 00173 ELA-MAX 00176 PREDNISOLONE ACETATE 00179 COLLOID SILVER 00184 KEPPRA 00187 OPHTHALMIC DROPS 00190 ABDEC 00191 HAPONAL 00192 SPECTRAVITE 00198 ENOXAPARIN SODIUM 00206 ACTONEL 00208 CELECOXIB 00209 GLUCOVANCE 00211 LEVALL 5.0 00213 PANTOPRAZOLE SODIUM 00217 TEMODAR 00218 CARBAMIDE PEROXIDE 00221 CHINESE HERBAL MEDS 00224 MILK AND MOLASSES ENEMA 00238 ZOLMITRIPTAN 00239 -

Johnson & Johnson's Janssen COVID-19 Vaccine Info
Johnson & Johnson’s Janssen COVID-19 Vaccine: Is It Efficacious? Purpose There has been observed brand hesitancy between the Johnson & Johnson Janssen COVID-19 vaccine as compared to the Pfizer and Moderna COVID-19 vaccine where individuals have expressed concerns over disparities in efficacy. This likely stems from the blanket efficacy statements reported in media outlets where the Johnson & Johnson’s vaccine reports an efficacy of 72% compared to 94% & 95% for Moderna and Pfizer respectively. The purpose of this info-bite is to clarify the concerns over efficacy so we can reassure our patients that all three vaccination options are efficacious and safe. The goal is to reduce vaccine brand hesitancy, as it may lead to a delay in vaccination, which may put our patients at risk. Is the Johnson & Johnson’s Janssen vaccine efficacious? Yes, the Johnson & Johnson Janssen vaccine has proven efficacy. Its reportedly 100% efficacious against death due to COVID-19 AND prevention against hospitalization. Additionally, it is potentially effective against variant strains as it maintained high efficacy against severe disease (73-82%) across world regions. The 72% efficacy often noted includes prevention of mild & moderate disease; it is unclear at this time how efficacious the vaccine is against mild & moderate disease. Is the Johnson & Johnson’s Janssen vaccine less efficacious than the Pfizer and Moderna vaccine? There is no evidence to claim that any of the three vaccines available are more efficacious than another. None of these vaccines have been compared in a head-to-head trial. Additionally, since the vaccine trials were tested at different times and locations, it further reduces one’s ability to claim one is better than the other. -
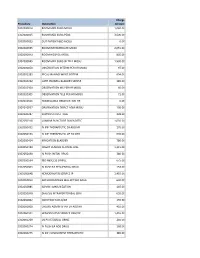
Procedure Description Charge Amount 0302000014 ROOM MED
Charge Procedure Description Amount 0302000014 ROOM MED SURG MOSU 1,040.00 0302000015 ROOM MED SURG PEDS 3,500.00 0302000033 OUT PATIENT BED MOSU 0.00 0302000035 ROOM INTERMEDIATE MOSU 2,055.00 0302000043 ROOM HOSPICE MOSU 805.00 0302000045 ROOM MED SURG W TELE MOSU 1,530.00 0302000050 OBSERVATION INTERM PER HR MOSU 97.00 0302002283 FECAL MANAGEMENT SYSTEM 694.00 0302010232 CATH INDWELL BLADDER SIMPLE 185.00 0302010304 OBSERVATION MS PER HR MOSU 60.00 0302010305 OBSERVATION TELE PER HR MOSU 71.00 0302010556 NONBILLABLE OBSERVATION HR 0.00 0302010557 OBSERVATION DIRECT ADM MOSU 130.00 0302020287 SUPPLIES CHEST TUBE 228.00 0302050318 LUMBAR PUNCTURE DIAGNOSTIC 1,035.00 0302050451 IV INF THERAPEUTIC EA ADD HR 170.00 0302050453 IV INF THERAPEUTIC UP TO 1HR 635.00 0302050454 IRRIGATION BLADDER 780.00 0302050480 INSERT VENOUS CENTRAL LINE 1,315.00 0302050490 IV PUSH INITIAL DRUG 380.00 0302050534 I&D ABSCESS SIMPLE 675.00 0302050603 IV PUSH EA SEQUENTIAL DRUG 153.00 0302050648 HEMODIALYSIS SERVICE IP 1,455.00 0302050813 ARTHROCENTESIS MAJ JNT WO IMAG 630.00 0302050885 ADMIN IMMUNIZATION 145.00 0302050948 DIALYSIS INTRAPERITONEAL SERV 655.00 0302060002 INJECTION SUB-Q/IM 155.00 0302060008 CHEMO ADMIN IV INF EA ADD HR 410.00 0302060101 HEMODIALYSIS SERVICE OBS/OP 1,455.00 0302060269 US PV RESIDUAL URINE 240.00 0302060274 IV PUSH EA ADD DRUG 168.00 0302060275 IV INF CONCURRENT THERAPEUTIC 385.00 0302060276 IV INF SEQUENTIAL THER UP TO 1 191.00 0302060293 INSERT STRAIGHT CATH THERAPEUT 185.00 0302060372 CHEMO ADMIN IV INF SEQ 1 HR 525.00 0302060373 -

2020 Over the Counter (OTC) Benefit Quick Reference Guide
2020 Community Health Choice (HMO D-SNP) OVER-THE-COUNTER (OTC) BENEFIT QUICK REFERENCE GUIDE Texas: Austin, Brazoria, Chambers, Fort Bend, Galveston, Hardin, Harris, Jasper, Jefferson, Liberty, Matagorda, Montgomery, Newton, Orange, Polk, San Jacinto, Tyler, Walker, Waller, and Wharton CommunityHealthChoice.org/Medicare 833.276.8306 or 713.295.5007 (TTY 711) October 1 through March 31, 8:00 am to 8:00 pm, 7 days a week and April 1 through September 30, Monday through Friday, 8:00 am to 8:00 pm H9826_PH_10163_111919_C 2020 Community Health Choice (HMO D-SNP) OVER-THE-COUNTER (OTC) BENEFIT QUICK REFERENCE GUIDE Plan Service Area CommunityHealthChoice.org/Medicare 833.276.8306 or 713.295.5007 (TTY 711) October 1 through March 31, 8:00 am to 8:00 pm, 7 days a week and April 1 through September 30, Monday through Friday, 8:00 am to 8:00 pm H9826_PH_10163_111919_C Thank you for choosing to get your Medicare health care and your prescription drug coverage through our plan, Community Health Choice (HMO D-SNP). As a Community Health Choice member, How do I use the OTC benefit? Over-The-Counter (OTC) Benefits Over-The-Counter (OTC) you get a quarterly Over-the-Counter As a member of Community Health Choice, (OTC) benefit that allows you to purchase you may obtain qualified OTC products items such as cold and cough medicines, through any pharmacy. After you have vitamins, dental care items, pain relievers, purchased your OTC products, you will and much more. The information below need to submit copies of your itemized describes how to take advantage of this purchase receipts within 30 days after the benefit.