Mcneil Consumer : Mdl No
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2015 Annual Report
ANNUAL REPORT 2015 MARCH 2016 TO OUR SHAREHOLDERS ALEX GORSKY Chairman, Board of Directors and Chief Executive Officer This year at Johnson & Johnson, we are proud this aligned with our values. Our Board of WRITTEN OVER to celebrate 130 years of helping people Directors engages in a formal review of 70 YEARS AGO, everywhere live longer, healthier and happier our strategic plans, and provides regular OUR CREDO lives. As I reflect on our heritage and consider guidance to ensure our strategy will continue UNITES & our future, I am optimistic and confident in the creating better outcomes for the patients INSPIRES THE long-term potential for our business. and customers we serve, while also creating EMPLOYEES long-term value for our shareholders. OF JOHNSON We manage our business using a strategic & JOHNSON. framework that begins with Our Credo. Written OUR STRATEGIES ARE BASED ON over 70 years ago, it unites and inspires the OUR BROAD AND DEEP KNOWLEDGE employees of Johnson & Johnson. It reminds OF THE HEALTH CARE LANDSCAPE us that our first responsibility is to the patients, IN WHICH WE OPERATE. customers and health care professionals who For 130 years, our company has been use our products, and it compels us to deliver driving breakthrough innovation in health on our responsibilities to our employees, care – from revolutionizing wound care in communities and shareholders. the 1880s to developing cures, vaccines and treatments for some of today’s most Our strategic framework positions us well pressing diseases in the world. We are acutely to continue our leadership in the markets in aware of the need to evaluate our business which we compete through a set of strategic against the changing health care environment principles: we are broadly based in human and to challenge ourselves based on the health care, our focus is on managing for the results we deliver. -

Drug Information Center Highlights of FDA Activities
Drug Information Center Highlights of FDA Activities – 9/1/20 – 9/30/20 FDA Drug Safety Communications & Drug Information Updates: Efficacy & Safety Concerns for Atezolizumab in Combination with Paclitaxel 9/8/20 The FDA alerted health care professionals and patients that a clinical trial evaluating atezolizumab plus paclitaxel in patients with previously untreated inoperable locally advanced or metastatic triple negative breast cancer that the drug combination was not effective. The combination of atezolizumab with another paclitaxel formulation, paclitaxel protein‐bound, is currently approved for use in adult patients with metastatic triple negative breast cancer, but this continued approval may be contingent on the results of additional studies. Paclitaxel should NOT be used as a replacement for paclitaxel protein bound in clinical practice. Electronic Expanded Access Requests 9/23/20 The FDA announced that the Reagan‐Udall Foundation has launched Expanded Access eRequest, a tool to submit expanded access requests for individual patient expanded access for drugs and biologics in non‐emergency settings. The tool allows auto population of forms, uploading of relevant documents, links to resources for physicians, patients, and caregivers, and secure application submission to the FDA. Benzodiazepine Drug Class: Drug Safety Communication ‐ Boxed Warning Update 9/23/20 The FDA is requiring the Boxed Warning be updated for all benzodiazepines to address serious risks of abuse, addiction, physical dependence, and withdrawal across the medication class. Changes are also being incorporated in the Medication Guides, and other sections of the prescribing information including the Warnings and Precautions, Drug Abuse and Dependence, and Patient Counseling Information sections. Diphenhydramine (Benadryl): Serious Problems with High Doses 9/24/20 The FDA issued a warning that taking higher than recommended doses of the common OTC allergy medication diphenhydramine (Benadryl) can lead to serious heart problems, seizures, coma, or even death. -

No. 33981 2 No
Pretoria, 4 February 2011 Februarle No. 33981 2 No. 33981 GOVERNMENT GAZETTE, 4 FEBRUARY 2011 IMPORTANT NOTICE The Government Printing Works will not be held responsible for faxed documents not received due to errors on the fax machine or faxes received which are unclear or incomplete. Please be advised that an "OK" slip, received from a fax machine, will not be accepted as proof that documents were received by the GPW for printing. If documents are faxed to the GPW it will be the sender's respon sibility to phone and confirm that the documents were received in good order. Furthermore the Government Printing Works will also not be held responsible for cancellations and amendments which have not been done on original documents received from clients. CONTENTS INHOUD Bladsy Koerant Page Gazette No. No. No. No. No. No. GENERAL NOTICE ALGEMENEKENNISGEWING Health, Department of Gesondheld, Departement van General Notice A/gemene Kennisgewing 58 Medicines and Related Substances Act 58 Wet op Beheer van Medisyne en (101/1965): Medicines Control Council: Verwante Stowwe (101/1965): Conditions of registration of a medicine Medisynebeheerraad: Voorwaardes vir in terms of the provisions of section die registrasie van 'n medisyne in terme 15 (7) ..................................................... .. 3 33981 van die bepalings van artikel 15 (7) ........ 4 33981 STAATSKOERANT, 4 FEBRUARIE 2011 No. 33981 3 GENERAL NOTICE ALGEMENE KENNISGEWING NOTICE 58 OF 2011 MEDICINES CONTROL COUNCIL CONDITIONS OF REGISTRATION OF A MEDICINE IN TERMS OF THE PROVISIONS OF SECTION 15(7) OF THE MEDICINES AND RELATED SUBSTANCES ACT, 1965 (ACT 101 OF 1965) 1. The applicant shall ensure that the medicine is manufactured and controlled in terms of the current Good Manufacturing Practices as determined by Council 2. -

Approved Prenatal Medications Pain Medications • Tylenol
Approved Prenatal Medications Pain Medications Tylenol (acetaminophen) for minor aches and pains, headaches. (Do not use: Aspirin, Motrin, Advil, Aleve, Ibuprofen.) Coughs/Colds Robitussin (Cough) Robitussin DM (non-productive cough) DO NOT USE TILL OVER 12 WEEKS Secrets and Vicks Throat Lozenges Mucinex Sore Throat Chloraseptic spray Saline Gargle Sucrets and Vicks Throat Lozenges Antihistamines/Allergies Zyrtec Claritin Benadryl Dimetapp Insomnia Benadryl Unison Hemorrhoids Preparation H Tucks Anusol Diarrhea Imodium (1-2 doses- if it persists please notify the office) BRAT diet (bananas, rice, applesauce, toast) Lice RID (only!) DO NOT USE Kwell Itching Benadryl Calamine or Caladryl Lotion Hydrocortisone cream Heartburn, Indigestion, Gas Tums Gas-X Mylanta Pepcid Maalox Zantac *DO NOT USE PEPTO BISMOL- it contains aspirin Decongestants Sudafed Robitussin CF- Only if over 12 weeks Tavist D Ocean Mist Nasal Spray (saline solutions) Nausea Small Frequent Meals Ginger Ale Vitamin B6 Sea Bands Yeast Infections Monistat Mycolog Gyne-lotrimin Toothache Orajel May see dentists, have cavity filled using Novocain or lidocaine, have x-rays with double lead shield, may have antibiotics in the Penicillin family (penicillin, amoxicillin) Sweetners- all should be consumed in moderation with water being consumed more frequently Nutrisweet (aspartame) Equal (aspartame) Splenda (sucralose) Sweet’n Low (saccharin) *note avoid aspartame if you have phenylketonuria (PKU) Constipation Colace Fibercon Citrucel Senokot Metamucil Milk of Magnesia Fiberall Miralax Eczema Hydrocortisone Cream Medications to AVOID Accurate Lithium Paxil Ciprofloxacin Tetracycline Coumadin Other Chemicals to AVOID Cigarettes Alcohol Recreational Drugs: marijuana, cocaine, ecstasy, heroin . -

Over-The-Counter (OTC) Medications Applies To: Tufts Health Ritogether and Tufts Health Together*
Over-the-Counter (OTC) Medications Applies to: Tufts Health RITogether and Tufts Health Together* As communicated in the November 1, 2018 Provider Update, the following changes are effective for fill dates on or after January 1, 2019. As a result of this change, some OTC medications will require prior authorization in certain circumstances as outlined below: Brand-Name OTC Medication Has a Covered Interchangeable Generic Version Available Afrin No Drip Advil capsule Advil tablet Advil PM tablet Afrin Nasal Spray Original nasal solution Afrin No Drip Aveeno Oatmeal Severe nasal Aleve tablet Baciguent ointment Benadryl capsule Bath Pak Treatment solution Benadryl Allergy Benadryl Allergy Benadryl Allergy Benadryl Extra Benefiber powder tablet capsule Liquid Strength cream Caltrate 600 +D Centrum Silver Betadine Swabstick Caltrate + D tablet Centrum liquid Plus Minerals tablet tablet Centrum Silver Centrum Ultra Men’s Children’s Advil Centrum tablet Cheracol-D syrup Adult 50+ tablet tablet suspension Children’s Benadryl Citracal Calcium + Children’s Benadryl Children’s Tylenol Chlor-Trimeton Allergy chewable D Slow Release Allergy liquid suspension syrup tablet tablet Citrucel Fiber Claritin-D 12 hour Claritin-D 24 hour Clear Cough Liquid Citrucel tablet Laxative powder tablet tablet PM Dimetapp DM Dex4 Fast Acting Dimetapp Cold and Colace capsule Conceptrol 4% gel Cough and Cold Glucose liquid Allergy elixir elixir Dristan nasal spray Dulcolax tablet D-Vi-Sol liquid Ecotrin tablet Evac powder Ex-Lax chewable Gas-X chewable Feosol tablet -

Notice Under S66 of the Commerce Act 1986 Application by Johnson & Johnson to Acquire the Stock, Assets and Business Of
PUBLIC COPY Notice under s66 of the Commerce Act 1986 Application by Johnson & Johnson to acquire the stock, assets and business of the Consumer Healthcare division of Pfizer Inc. COMMERCE ACT 1986: BUSINESS ACQUISITION SECTION 66: NOTICE SEEKING CLEARANCE 28 September 2006 The Registrar Business Acquisitions and Authorisations Commerce Commission PO Box 2351 Wellington Pursuant to s66(1) of the Commerce Act 1986 notice is hereby given seeking clearance of a proposed business acquisition. 518689_1.DOC 2 CONTENTS EXECUTIVE SUMMARY PART 1: TRANSACTION DETAILS 1 The business acquisition for which clearance is sought 2 The person giving this notice 3 Confidentiality 4 Participants 5 Interconnected and associated persons 6 Beneficial interests 7 Links between participants 8 Common directorships 9 Business activities of the participants 10 Reasons for the proposed acquisition PART II: IDENTIFICATION OF MARKETS AFFECTED 11 Horizontal aggregation 12 Differentiated product markets 13 Differentiated product markets 14 Vertical integration 15 Other business acquisitions PARTS III, IV AND V: CONSTRAINTS ON MARKET POWERS BY EXISTING AND POTENTIAL COMPETITION AND OTHER POTENTIAL CONSTRAINTS 16 Allergy medication 17 Products for the treatment of worms 18 Thrush treatment CERTIFICATE APPENDICES 1. Heartburn and indigestion remedies (MYLANTA and MOTILIUM) 2. Worm treatments (COMBANTRIN, VERMOX) 3. Cold, flu, nasal decongestant, cough relief and sort throat medications (CODRAL, SINUTAB, SUDAFED, BENADRYL, BRONDECON) 4. Allergy relief products (ACTIFED, SINUTAB, SUDAFED, VISINE, LIVOSTIN) 518689_1.DOC 3 5. Thrush treatments (DIFLUCAN ONE, DAKTARIN, DAKTAGOLD, NIZORAL, SPORANOX) 6. Shampoo (PREGAINE, ROGAINE, NEUTROGENA, JOHNSON’S BABY SHAMPOO) 7. Hand hygiene (PURELL and MICRO SHIELD) 8. Competitor worm treatment products 9. Multinational pharmaceutical businesses: GlaxoSmithKline, Douglas Pharmaceuticals, Alphapharm, Bayer Group 10. -

Benadryl (Diphenhydramine) Dosing Chart
1084 Cromwell Avenue Rocky Hill, CT 06067 (860) 721-7561 – Phone (860) 721-9199 – Fax Benadryl (Diphenhydramine) Dosing Chart *Ages 6 months & above* Dose every 6 hours: Dose for hives and/or itching. In general, once hives have started, you should continue regular dosing until there have not been hives for the entire day. Benadryl (Diphenhydramine) Liquid: Chewable Tablets: Tablets: Weight: 12.5mg/5ml 12.5mg each 25 mg each 17-21 pounds ¾ tsp. (3.75ml) Use Liquid Use Liquid Use 22-32 pounds 1 tsp. (5ml) 1 tablet Liquid or Chews Use 33-42 pounds 1 ½ tsp. (7.5ml) 1 ½ tablets Liquid or Chews 43-53 pounds 2 tsp. (10ml) 2 tablets 1 tablet 54-64 pounds 2 ½ tsp. (12.5ml) 2 ½ tablets 1 tablet 65-75 pounds 3 tsp. (15ml) 3 tablets 1 tablet 76-86 pounds 3 ½ tsp. (17.5ml) 3 ½ tablets 1 tablet >86 pounds 4 tsp. (20ml) 4 tablets 2 tablets EDUCATION ON CALL Administering Medicine Safely When your child isn’t feeling well, you want to relieve their discomfort as quickly as possible. Be prepared with information that can help you understand the differences between pediatric pain relievers and fever reducers, and how to administer them safely. Always read the label 1. Active ingredient: Ingredient that makes the medicine work 2. Uses: Symptoms the medicine treats 3. Directions: The amount of medicine to give and how often Know the difference TYLENOL® MOTRIN® Active ingredient: Acetaminophen Active ingredient: Ibuprofen • Treats pain & fever • Treats pain & fever • Gentle on tummies • Lasts up to 8 hours • Dosing available from your pediatrician for • Can be used for children 6 months of age or older children 6 months and younger Never give aspirin to children. -
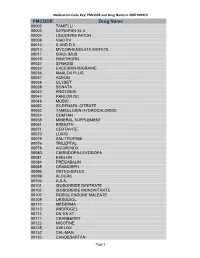
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS Cdc-Pdf
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00002 TAMIFLU 00003 DITROPAN XL II 00004 LIDODERM PATCH 00008 VIACTIV 00010 A AND D II 00013 MYCOPHENOLATE MOFETIL 00017 SIROLIMUS 00019 HAWTHORN 00027 SYNAGIS 00032 EXCEDRIN MIGRAINE 00036 MAALOX PLUS 00037 ACEON 00038 GLYSET 00039 SONATA 00042 PROTONIX 00044 PANLOR DC 00048 MOBIC 00052 SILDENAFIL CITRATE 00053 TAMSULOSIN HYDROCHLORIDE 00054 COMTAN 00058 MINERAL SUPPLEMENT 00061 BISMUTH 00071 CERTAVITE 00073 LUXIQ 00075 SAL-TROPINE 00076 TRILEPTAL 00078 AGGRENOX 00080 CARBIDOPA-LEVODOPA 00081 EXELON 00084 PREGABALIN 00085 ORAMORPH 00096 OSTEO-BIFLEX 00099 ALOCRIL 00100 A.S.A. 00101 ISOSORBIDE DINITRATE 00102 ISOSORBIDE MONONITRATE 00107 ROSIGLITAZONE MALEATE 00109 URSODIOL 00112 MEDERMA 00113 ANDROGEL 00114 DILTIA XT 00117 CRANBERRY 00123 NICOTINE 00125 AVELOX 00132 CAL-MAG 00133 CANDESARTAN Page 1 Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00148 PROLIXIN D 00149 D51/2 NS 00150 NICODERM CQ PATCH 00151 TUSSIN 00152 CEREZYME 00154 CHILDREN'S IBUPROFEN 00156 PROPOXACET-N 00159 KALETRA 00161 BISOPROLOL 00167 NOVOLIN N 00169 KETOROLAC TROMETHAMINE 00172 OPHTHALMIC OINTMENT 00173 ELA-MAX 00176 PREDNISOLONE ACETATE 00179 COLLOID SILVER 00184 KEPPRA 00187 OPHTHALMIC DROPS 00190 ABDEC 00191 HAPONAL 00192 SPECTRAVITE 00198 ENOXAPARIN SODIUM 00206 ACTONEL 00208 CELECOXIB 00209 GLUCOVANCE 00211 LEVALL 5.0 00213 PANTOPRAZOLE SODIUM 00217 TEMODAR 00218 CARBAMIDE PEROXIDE 00221 CHINESE HERBAL MEDS 00224 MILK AND MOLASSES ENEMA 00238 ZOLMITRIPTAN 00239 -
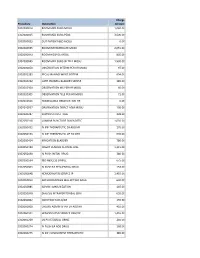
Procedure Description Charge Amount 0302000014 ROOM MED
Charge Procedure Description Amount 0302000014 ROOM MED SURG MOSU 1,040.00 0302000015 ROOM MED SURG PEDS 3,500.00 0302000033 OUT PATIENT BED MOSU 0.00 0302000035 ROOM INTERMEDIATE MOSU 2,055.00 0302000043 ROOM HOSPICE MOSU 805.00 0302000045 ROOM MED SURG W TELE MOSU 1,530.00 0302000050 OBSERVATION INTERM PER HR MOSU 97.00 0302002283 FECAL MANAGEMENT SYSTEM 694.00 0302010232 CATH INDWELL BLADDER SIMPLE 185.00 0302010304 OBSERVATION MS PER HR MOSU 60.00 0302010305 OBSERVATION TELE PER HR MOSU 71.00 0302010556 NONBILLABLE OBSERVATION HR 0.00 0302010557 OBSERVATION DIRECT ADM MOSU 130.00 0302020287 SUPPLIES CHEST TUBE 228.00 0302050318 LUMBAR PUNCTURE DIAGNOSTIC 1,035.00 0302050451 IV INF THERAPEUTIC EA ADD HR 170.00 0302050453 IV INF THERAPEUTIC UP TO 1HR 635.00 0302050454 IRRIGATION BLADDER 780.00 0302050480 INSERT VENOUS CENTRAL LINE 1,315.00 0302050490 IV PUSH INITIAL DRUG 380.00 0302050534 I&D ABSCESS SIMPLE 675.00 0302050603 IV PUSH EA SEQUENTIAL DRUG 153.00 0302050648 HEMODIALYSIS SERVICE IP 1,455.00 0302050813 ARTHROCENTESIS MAJ JNT WO IMAG 630.00 0302050885 ADMIN IMMUNIZATION 145.00 0302050948 DIALYSIS INTRAPERITONEAL SERV 655.00 0302060002 INJECTION SUB-Q/IM 155.00 0302060008 CHEMO ADMIN IV INF EA ADD HR 410.00 0302060101 HEMODIALYSIS SERVICE OBS/OP 1,455.00 0302060269 US PV RESIDUAL URINE 240.00 0302060274 IV PUSH EA ADD DRUG 168.00 0302060275 IV INF CONCURRENT THERAPEUTIC 385.00 0302060276 IV INF SEQUENTIAL THER UP TO 1 191.00 0302060293 INSERT STRAIGHT CATH THERAPEUT 185.00 0302060372 CHEMO ADMIN IV INF SEQ 1 HR 525.00 0302060373 -

Nevada Medicaid Formulary
Nevada Medicaid-Approved Preferred Drug List Effective August 15, 2021 Legend In each class, drugs are listed alphabetically by either brand name or generic name. Brand name drug: Uppercase in bold type Generic drug: Lowercase in plain type AL: Age Limit Restrictions DO: Dose Optimization Program GR: Gender Restriction OTC: Over the counter medication available with a prescription. (Prescribers please indicate OTC on the prescription) PA: Prior authorization is required. Prior authorization is the process of obtaining approval of benefits before certain prescriptions are filled. QL: Quantity limits; certain prescription medications have specific quantity limits per prescription or per month. SP: Specialty Pharmacy ST: Step therapy is required. You may need to use one medication before benefits for the use of another medication can be authorized. Drug Name Reference Notes *ADHD/ANTI-NARCOLEPSY/ANTI- OBESITY/ANOREXIANTS* *ADHD AGENT - SELECTIVE ALPHA ADRENERGIC AGONISTS*** clonidine hcl er oral tablet extended release Kapvay AL; QL 12 hour *ADHD AGENT - SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR*** atomoxetine hcl oral capsule Strattera DO; AL; QL *AMPHETAMINE MIXTURES*** amphetamine-dextroamphet er oral capsule extended release 24 hour 10 mg, 15 mg, 5 Adderall XR DO; AL; QL mg amphetamine-dextroamphet er oral capsule extended release 24 hour 20 mg, 25 mg, 30 Adderall XR AL; QL mg amphetamine-dextroamphetamine oral Adderall DO; AL; QL tablet 10 mg, 12.5 mg, 15 mg, 5 mg, 7.5 mg amphetamine-dextroamphetamine oral Adderall AL tablet 20 mg, -

Over-The-Counter (OTC) Medications
Over-the-Counter (OTC) Medications Helpful Tips • Choose products that target your specific symptoms, rather than combination products that treat many symptoms that you may not have. See below. • Be cautious of doubling up on the same ingredients (for example, Tylenol is in many products). • Take extra care when using “PM” products, as they may have serious side effects. • Always read the product labels for important dosing and precaution information. - Doses may differ for adults and children - Many products have age limitations - Most products have a maximum number of days that they should be used OverO-thever--counterthe-counter options options Over-the-Counter Options CombinationCombination Products: Products: treat multipletreat multiple symptoms symptoms Combination products often results in overtreating because you’re taking medications for Combination Products: Treat multiple symptoms Combination• products often results in overtreating because you’re taking medications for • symptoms that you do not have. • Combination products often result in overtreating because you’re taking medications for symptoms that you do not have. Instead,Over choose-the- counteran over-the options-counter p roduct that targets your specific symptoms with symptoms that you do not have. •Instead, choose an over-the-counter product that targets your specific symptoms with • ingredients only for those symptom • Instead, choose an over-the-counter product that targets your specific symptoms with Combination Products:ingredients treat multipleonly for thosesymptoms -

Transdermal Nicotine Maintenance Attenuates the Subjective And
Neuropsychopharmacology (2004) 29, 991–1003 & 2004 Nature Publishing Group All rights reserved 0893-133X/04 $25.00 www.neuropsychopharmacology.org Transdermal Nicotine Maintenance Attenuates the Subjective and Reinforcing Effects of Intravenous Nicotine, but not Cocaine or Caffeine, in Cigarette-Smoking Stimulant Abusers 1 1 ,1,2 Bai-Fang X Sobel , Stacey C Sigmon and Roland R Griffiths* 1Department of Psychiatry and Behavioral Science, Johns Hopkins University School of Medicine, Baltimore, MD, USA; 2Department of Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD, USA The effects of transdermal nicotine maintenance on the subjective, reinforcing, and cardiovascular effects of intravenously administered cocaine, caffeine, and nicotine were examined using double-blind procedures in nine volunteers with histories of using tobacco, caffeine, and cocaine. Each participant was exposed to two chronic drug maintenance phases (21 mg/day nicotine transdermal patch and placebo transdermal patch). Within each drug phase, the participant received intravenous injections of placebo, cocaine (15 and 30 mg/70 kg), caffeine (200 and 400 mg/70 kg), and nicotine (1.0 and 2.0 mg/70 kg) in mixed order across days. Subjective and cardiovascular data were collected before and repeatedly after drug or placebo injection. Reinforcing effects were also assessed after each injection with a Drug vs Money Multiple-Choice Form. Intravenous cocaine produced robust dose-related increases in subjective and reinforcing effects; these effects were not altered by nicotine maintenance. Intravenous caffeine produced elevations on several subjective ratings; nicotine maintenance did not affect these ratings. Under the placebo maintenance condition, intravenous nicotine produced robust dose-related subjective effects, with maximal increases similar to the high dose of cocaine; nicotine maintenance significantly decreased the subjective and reinforcing effects of intravenous nicotine.