No. 33981 2 No
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mcneil Consumer : Mdl No
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA IN RE: MCNEIL CONSUMER : MDL NO. 2190 HEALTHCARE, ET AL., MARKETING : AND SALES PRACTICES LITIGATION : : Applies to: : ALL ACTIONS : MEMORANDUM McLaughlin, J. July 13, 2012 This multidistrict litigation arises out of quality control problems at the defendants’ facility manufacturing over- the-counter healthcare products in Fort Washington, Pennsylvania, which led to a series of recalls of those products. The named plaintiffs assert claims for economic loss on behalf of a putative nationwide class against Johnson & Johnson (“J&J”), McNeil Consumer Healthcare (“McNeil”), and four of their executives. The plaintiffs allege that they overpaid for the defendants’ products as a result of the recalls and the defendants’ scheme to conceal or downplay the scope of the quality control problems. The defendants, who have offered a coupon or cash refund to consumers who purchased recalled drugs, have moved to dismiss the operative complaint, and assert that the named plaintiffs lack constitutional standing and have not met the applicable pleading standard. The Court will grant the defendants’ motion because the plaintiffs have not pled facts that show a cognizable injury in fact, which is required to confer Article III standing. I. Procedural Background This litigation resulted from the consolidation of ten individual actions filed around the country. Haviland v. McNeil Consumer Healthcare, No. 10-2195, was filed in this Court on May 12, 2010, asserting economic injuries arising out of the April 30, 2010 recall of over-the-counter children’s drugs by McNeil, a part of the J&J “Family of Companies.” Eight additional cases, also arising out of the April 2010 recall, were filed in district courts around the country.1 All cases asserted claims for economic injury only, with the exception of Rivera v. -

IN the UNITED STATES DISTRICT COURT for the DISTRICT of DELAWARE ALZA CORPORATION and JANSSEN PHARMACEUTICALS, INC., Plaintiffs
Case 1:16-cv-00914-UNA Document 3 Filed 10/07/16 Page 1 of 20 PageID #: 24 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE ALZA CORPORATION and ) JANSSEN PHARMACEUTICALS, INC., ) ) Plaintiffs, ) ) v. ) Civil Action No. ____________ ) REDACTED PUBLIC VERSION AMNEAL PHARMACEUTICALS OF ) NEW YORK, LLC and ) AMNEAL PHARMACEUTICALS LLC, ) ) Defendants. ) COMPLAINT In this patent infringement action, Plaintiffs ALZA Corporation ("ALZA") and Janssen Pharmaceuticals, Inc. (collectively "Plaintiffs"), for their complaint against Defendants Amneal Pharmaceuticals of New York, LLC ("Amneal Pharms. NY") and Amneal Pharmaceuticals LLC ("Amneal Pharms. LLC") (collectively, "Amneal"), allege as follows: NATURE OF THE ACTION 1. This is an action for patent infringement under the patent laws of the United States, Title 35, United States Code, in response to, inter alia, the submission by Amneal of Abbreviated New Drug Application ("ANDA") No. 207515, with the U.S. Food and Drug Administration ("FDA") seeking approval to manufacture and sell a generic version of CONCERTA® prior to the expiration of U.S. Patent No. 8,163,798 ("the '798 patent") and U.S. Patent No. 9,144,549 ("the '549 patent"). PARTIES 2. Plaintiff ALZA is a Delaware corporation, having its principal place of business at 700 Eubanks Drive, Vacaville, California 95688. Case 1:16-cv-00914-UNA Document 3 Filed 10/07/16 Page 2 of 20 PageID #: 25 REDACTED PUBLIC VERSION 3. Plaintiff Janssen Pharmaceuticals, Inc. ("Janssen") is a Pennsylvania corporation, having a place of business at 1125 Trenton-Harbourton Road, Titusville, New Jersey 08560. 4. On information and belief, Defendant Amneal Pharms. LLC is a limited liability company organized under the laws of the State of Delaware and has a place of business at 400 Crossing Boulevard, Bridgewater, NJ 08807. -

Management Team
Management Team Bruce C. Cozadd Executive Chairman Bruce Cozadd joined Jazz Pharmaceuticals at its inception. From 2001 until he joined Jazz Pharmaceuticals, Mr. Cozadd served as a consultant to companies in the biopharmaceutical industry. From 1991 until 2001, he held various positions with ALZA Corporation, a pharmaceutical company now owned by Johnson & Johnson, most recently as its Executive Vice President and Chief Operating Officer, with responsibility for research and development, manufacturing and sales and marketing. Previously at ALZA Corporation he held the roles of Chief Financial Officer and Vice President, Corporate Planning and Analysis. Mr. Cozadd received a B.S. from Yale University and an M.B.A. from the Stanford Graduate School of Business. Mr. Cozadd serves on the boards of Cerus Corporation, a biopharmaceutical company; Threshold Pharmaceuticals, a biotechnology company; and The Nueva School and Stanford Hospital and Clinics, both non-profit organizations. Samuel R. Saks, MD Chief Executive Officer Samuel Saks, M.D., joined Jazz Pharmaceuticals at its inception. From 2001 until he joined Jazz Pharmaceuticals, Dr. Saks was Company Group Chairman of ALZA Corporation and served as a member of the Johnson & Johnson Pharmaceutical Group Operating Committee. From 1992 until 2001, he held various positions with ALZA Corporation, most recently as its Chief Medical Officer and Group Vice President, where he was responsible for clinical and commercial activities. Dr. Saks received a B.S. and an M.D. from the University of Illinois. Dr. Saks serves on the board of Trubion Pharmaceuticals and Cougar Biotechnology. Robert M. Myers President Robert Myers joined Jazz Pharmaceuticals at its inception and was appointed as Jazz Pharmaceuticals’ President in March 2007. -

Notice Under S66 of the Commerce Act 1986 Application by Johnson & Johnson to Acquire the Stock, Assets and Business Of
PUBLIC COPY Notice under s66 of the Commerce Act 1986 Application by Johnson & Johnson to acquire the stock, assets and business of the Consumer Healthcare division of Pfizer Inc. COMMERCE ACT 1986: BUSINESS ACQUISITION SECTION 66: NOTICE SEEKING CLEARANCE 28 September 2006 The Registrar Business Acquisitions and Authorisations Commerce Commission PO Box 2351 Wellington Pursuant to s66(1) of the Commerce Act 1986 notice is hereby given seeking clearance of a proposed business acquisition. 518689_1.DOC 2 CONTENTS EXECUTIVE SUMMARY PART 1: TRANSACTION DETAILS 1 The business acquisition for which clearance is sought 2 The person giving this notice 3 Confidentiality 4 Participants 5 Interconnected and associated persons 6 Beneficial interests 7 Links between participants 8 Common directorships 9 Business activities of the participants 10 Reasons for the proposed acquisition PART II: IDENTIFICATION OF MARKETS AFFECTED 11 Horizontal aggregation 12 Differentiated product markets 13 Differentiated product markets 14 Vertical integration 15 Other business acquisitions PARTS III, IV AND V: CONSTRAINTS ON MARKET POWERS BY EXISTING AND POTENTIAL COMPETITION AND OTHER POTENTIAL CONSTRAINTS 16 Allergy medication 17 Products for the treatment of worms 18 Thrush treatment CERTIFICATE APPENDICES 1. Heartburn and indigestion remedies (MYLANTA and MOTILIUM) 2. Worm treatments (COMBANTRIN, VERMOX) 3. Cold, flu, nasal decongestant, cough relief and sort throat medications (CODRAL, SINUTAB, SUDAFED, BENADRYL, BRONDECON) 4. Allergy relief products (ACTIFED, SINUTAB, SUDAFED, VISINE, LIVOSTIN) 518689_1.DOC 3 5. Thrush treatments (DIFLUCAN ONE, DAKTARIN, DAKTAGOLD, NIZORAL, SPORANOX) 6. Shampoo (PREGAINE, ROGAINE, NEUTROGENA, JOHNSON’S BABY SHAMPOO) 7. Hand hygiene (PURELL and MICRO SHIELD) 8. Competitor worm treatment products 9. Multinational pharmaceutical businesses: GlaxoSmithKline, Douglas Pharmaceuticals, Alphapharm, Bayer Group 10. -
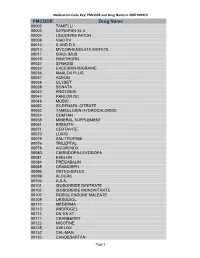
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS Cdc-Pdf
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00002 TAMIFLU 00003 DITROPAN XL II 00004 LIDODERM PATCH 00008 VIACTIV 00010 A AND D II 00013 MYCOPHENOLATE MOFETIL 00017 SIROLIMUS 00019 HAWTHORN 00027 SYNAGIS 00032 EXCEDRIN MIGRAINE 00036 MAALOX PLUS 00037 ACEON 00038 GLYSET 00039 SONATA 00042 PROTONIX 00044 PANLOR DC 00048 MOBIC 00052 SILDENAFIL CITRATE 00053 TAMSULOSIN HYDROCHLORIDE 00054 COMTAN 00058 MINERAL SUPPLEMENT 00061 BISMUTH 00071 CERTAVITE 00073 LUXIQ 00075 SAL-TROPINE 00076 TRILEPTAL 00078 AGGRENOX 00080 CARBIDOPA-LEVODOPA 00081 EXELON 00084 PREGABALIN 00085 ORAMORPH 00096 OSTEO-BIFLEX 00099 ALOCRIL 00100 A.S.A. 00101 ISOSORBIDE DINITRATE 00102 ISOSORBIDE MONONITRATE 00107 ROSIGLITAZONE MALEATE 00109 URSODIOL 00112 MEDERMA 00113 ANDROGEL 00114 DILTIA XT 00117 CRANBERRY 00123 NICOTINE 00125 AVELOX 00132 CAL-MAG 00133 CANDESARTAN Page 1 Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00148 PROLIXIN D 00149 D51/2 NS 00150 NICODERM CQ PATCH 00151 TUSSIN 00152 CEREZYME 00154 CHILDREN'S IBUPROFEN 00156 PROPOXACET-N 00159 KALETRA 00161 BISOPROLOL 00167 NOVOLIN N 00169 KETOROLAC TROMETHAMINE 00172 OPHTHALMIC OINTMENT 00173 ELA-MAX 00176 PREDNISOLONE ACETATE 00179 COLLOID SILVER 00184 KEPPRA 00187 OPHTHALMIC DROPS 00190 ABDEC 00191 HAPONAL 00192 SPECTRAVITE 00198 ENOXAPARIN SODIUM 00206 ACTONEL 00208 CELECOXIB 00209 GLUCOVANCE 00211 LEVALL 5.0 00213 PANTOPRAZOLE SODIUM 00217 TEMODAR 00218 CARBAMIDE PEROXIDE 00221 CHINESE HERBAL MEDS 00224 MILK AND MOLASSES ENEMA 00238 ZOLMITRIPTAN 00239 -
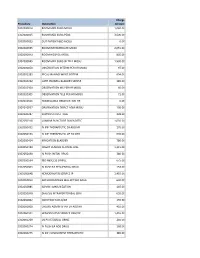
Procedure Description Charge Amount 0302000014 ROOM MED
Charge Procedure Description Amount 0302000014 ROOM MED SURG MOSU 1,040.00 0302000015 ROOM MED SURG PEDS 3,500.00 0302000033 OUT PATIENT BED MOSU 0.00 0302000035 ROOM INTERMEDIATE MOSU 2,055.00 0302000043 ROOM HOSPICE MOSU 805.00 0302000045 ROOM MED SURG W TELE MOSU 1,530.00 0302000050 OBSERVATION INTERM PER HR MOSU 97.00 0302002283 FECAL MANAGEMENT SYSTEM 694.00 0302010232 CATH INDWELL BLADDER SIMPLE 185.00 0302010304 OBSERVATION MS PER HR MOSU 60.00 0302010305 OBSERVATION TELE PER HR MOSU 71.00 0302010556 NONBILLABLE OBSERVATION HR 0.00 0302010557 OBSERVATION DIRECT ADM MOSU 130.00 0302020287 SUPPLIES CHEST TUBE 228.00 0302050318 LUMBAR PUNCTURE DIAGNOSTIC 1,035.00 0302050451 IV INF THERAPEUTIC EA ADD HR 170.00 0302050453 IV INF THERAPEUTIC UP TO 1HR 635.00 0302050454 IRRIGATION BLADDER 780.00 0302050480 INSERT VENOUS CENTRAL LINE 1,315.00 0302050490 IV PUSH INITIAL DRUG 380.00 0302050534 I&D ABSCESS SIMPLE 675.00 0302050603 IV PUSH EA SEQUENTIAL DRUG 153.00 0302050648 HEMODIALYSIS SERVICE IP 1,455.00 0302050813 ARTHROCENTESIS MAJ JNT WO IMAG 630.00 0302050885 ADMIN IMMUNIZATION 145.00 0302050948 DIALYSIS INTRAPERITONEAL SERV 655.00 0302060002 INJECTION SUB-Q/IM 155.00 0302060008 CHEMO ADMIN IV INF EA ADD HR 410.00 0302060101 HEMODIALYSIS SERVICE OBS/OP 1,455.00 0302060269 US PV RESIDUAL URINE 240.00 0302060274 IV PUSH EA ADD DRUG 168.00 0302060275 IV INF CONCURRENT THERAPEUTIC 385.00 0302060276 IV INF SEQUENTIAL THER UP TO 1 191.00 0302060293 INSERT STRAIGHT CATH THERAPEUT 185.00 0302060372 CHEMO ADMIN IV INF SEQ 1 HR 525.00 0302060373 -

TITLE Safety and Efficacy of Over-The-Counter Drug Use by the Elderly
" DOCUMENT RESUME ED 245 148 CG 017 518 TITLE Safety and Efficacy of Over-the-Counter Drug Use by the Elderly. Hearing before the Subcommittee on Health and Long-Term Care of the Select Committee on Aging. House of Representatives, Ninety-Eighth Congress, First Session. INSTITUTION Congress of the U.S., Washington, D.C. House Select Committee on Aging. REPORT NO House-Comm-Pub-98-409 PUB DATE 21 Jul 83 NOTE 507p.; Some pages are marginally legible due to small print. PUB TYPE Legal/Legislative/Regulatory Materials (090) EDRS PRICE MF02 Plus Postage. PC Not Available from EDRS. DESCRIPTORS *Consumer Protection; Dietetics; Drug Legislation; *Drug Use; Gerontology; Hearings; *Older Adults; Physical Health; *Safety IDENTIFIERS Congress 98th; Long Term Care; *Nonprescription Drugs; *PPA ABSTRACT This document contains the prepared statements and panel testimony from the Congressional hearing on over-the-counter (OTC) drug use by the elderly. Opening statements are given by Representatives Claude Pepper (chairman), Ralph Regula, Mary Rose Oakar, Michael Bilirakis, Tom Lantos, and Hal Daub. Topics which are covered include the incidence and quantity of drug use by the elderly, health risks, adverse reactions, phenylpropanolamine (PPA), consumer protection, and the Federal Drug Administration's (FDA) role in the OTC drug safety and reguIatiem. Testimony of the first panel on OTC drugs, particularly weight reduction medications containing PPA, is given by representatives of the Health Research Group, the National Broadcasting Company, and consumers. Testimony of the second panel on mail fraud schemes perpetrated against senior citizens is given by consumer advocates representing the United States Postal Service, Criminal Investigations and Consumer Protection Divisions, and the Center for Science in the Public Interest. -

Memorandum JUL 1 6 201D
Memorandum Subject Date Additional Quota Letters Received by July 15, 2010 (DFN: 630-08.2) JUL 1 6 201D To From Christine A. Sanncrud. Ph.D., Chief "Barbar• J.Illoockholdt, Chief Drug & Chemical Evaluation Section Regul• torn Section Office of Diverison Control Off cL rl Diversion Control On July 15. 2010, this section received your e-mail requesting a review of seventeen (17) quota applications from sixteen (16) registered manufacturers to determine if there arc any pending administrative/legal actions against these applicants and to advise ODE of the findings. ODOR conducted reviews (NADDIS, CSA, etc), as well as surveyed the responsible field offices for their input and recommendations. Provided below are the results and recommendations. QUOTA APPLICANTS wan NO ADVERSE OR DEROGATORY INFORMATIO N Novartis Consumer I lealth Lincoln (10345) (b)(4);(b)(7)(E) Baxter (10346) Generics Bidco li bda Vintage (10347) Noramco Delaware (10348) Pharmaceuticals International inc. (10349) Pharmedium (10351) Pharmedium (10352) Rhodes (10356) Bio-Pharm (10357) Patheon (10358) Patheon (10359) Watson (10361) B & B (10363) lospira. Inc. NC (10364) Epic Pharma (10366) Mallinckrodt I lobart (10367) Chemtos (10368) Vol. II Page 55 2 Per consultation with the field offices. DEA does not have sufficient grounds to limit, restrict. or deny quota requests from these registrants. Based on this information. ODG suggests that you proceed with the completion of the quota applications. If you have any questions pertaining to this information. please feel free to contact me (b)(6);(b)(7)( or SC C) (b)(6);(b)(7)(C) Vol. II Page 56 Memorandum Subject Date Additional Quota Letters Received as of July 19, 201() (DFN: 630-08.2) stir 2 8 2010,., To Fr ,./"./ Christine A. -

Management Liability Focus Johnson & Johnson 1
Insured Profile Report – Management Liability Focus Johnson_________________________________________________________________________ & Johnson Company Profile Credit Details Location 1 Johnson and Johnson Plz Overall Credit Risk High Risk New Brunswick, NJ www.jnj.com Number of Legal Derogatory 84 Company Type Public Items Liability Amount $322,285.00 Formerly Known As N/A Experian Intelliscore 2.57 SIC Code 2834 SIC Code Description Pharmaceutical Preparations Experian Intelliscore Percentile 2.00 % of companies score lower and have higher credit risk Established 1955 Experian Commercial IntelliscoreSM is an all-industry commercial model using business information to predict business risk. Its Sales (in millions) $65,030.00 predictiveness is among the best on the market today The objective of the Commercial Intelliscore Model is to predict seriously Employees 117,900 derogatory payment behavior. Possible score range from 0 to 100, where 0 is high risk and 100 is low risk Total OSHA Violations 18 -Liability Amount is the total dollar amount of debtor’s legal liability, OSHA is an arm of the Department of Labor that conducts inspections of company including accounts in collection, tax liens,judgments and/or bankruptcies facilities with the goal of preventing work-related injuries, illnesses and deaths. -The Number of Legal Derogatory items are the sum of Tax-Lien Worksites that do not meet health and/or safety standards at the time of inspection may Count, Bankruptcy,Judgment, Collection-Counter and UCC Derog receive an OSHA violation. Total FDA NDC Drugs 177 The total number of FDA Drugs filed in the FDA NDC Drug Database. Business Description Johnson & Johnson is engaged in the research and development, manufacture and sale of a range of products in the healthcare field. -

1851022002T Hydrocortisone Ointment 0.5% Mac's
List of Drugs Registered in Accordance with the provisions of the Food and Drugs Act and Regulations Chapter 30:01 Year 2002 DAN TRADE NAME AND MANUFACTURER COUNTRY CONDITION FORM OF OF SALE ORIGIN 1851022002T Hydrocortisone Ointment Mac’s Pharmaceuticals Jamaica Third Schedule 0.5% 1852022002T Hydrocortisone Ointment Mac’s Pharmaceuticals Jamaica Third Schedule 1% 1853022002T Hydrocortisone Cream Mac’s Pharmaceuticals Jamaica Third Schedule 0.5% 1854022002T Hydrocortisone Cream Mac’s Pharmaceuticals Jamaica Third Schedule 1% 1855022002 New-Glow Antifungal Mac’s Pharmaceuticals Jamaica Freely Solution 1856022002 Paradmol Tablets Mac’s Pharmaceuticals Jamaica Freely 1857022002 Paradmol Elixir Mac’s Pharmaceuticals & Jamaica Freely Cosmetics 1858022002 Paradmol Elixir - Alcohol Mac’s Pharmaceuticals & Jamaica Freely Free Cosmetics 1859022002T Cifran Injection Ranbaxy Laboratories India Third Schedule 200mg/100ml Limited 1739022002 Bell’s Antiseptic Cream Adams Healthcare England Freely England for Bell, Sons & Co (Druggists) Ltd. 1740022002 Menthodex Lozenges Bell, Sons & Co England Freely (Druggists) Ltd. 1741022002 Proactin Syrup Meyer Organics Ltd. India Freely 1742022002T Intaxel Injection Dabur India Ltd. India Third Schedule 100mg/17ml (Pharmaceutical Division) 1743022002T Topotel Injection Dabur India Ltd. India Third Schedule Concentrate 4mg (Pharmaceutical Division) DAN TRADE NAME AND MANUFACTURER COUNTRY CONDITION FORM OF OF SALE ORIGIN 1744022002T Cytarine Injection 100mg Dabur India Ltd. India Third Schedule (Pharmaceutical Division) 1745022002 HbRich Syrup Naxpar Lab. Pvt. Ltd. India Freely 1746022002 HbRich-300 Composite Wockhardt Limited India Freely Pack 1747022002 Juvelon Capsules Wockhardt Limited India Freely 1748022002T Aerobon Inhaler Wockhardt Limited India Third Schedule 100mcg/dose 1749022002 Ehrlich Balsam Joint Ehrlich Pharma Allgauer Germany Freely +Muscle Balsam Heilmoor Ehrlich GmbH 1750022002T FluaRIX Junior SSW Dresden a branch Germany Third Schedule Inactivated Split Influenza of SB Pharma GmbH & Vaccine - Suspension for Co. -

Annual Report
ANNUAL REPORT 2019 MARCH 2020 To Our Shareholders Alex Gorsky Chairman and Chief Executive Officer By just about every measure, Johnson & These are some of the many financial and Johnson’s 133rd year was extraordinary. strategic achievements that were made possible by the commitment of our more than • We delivered strong operational revenue and 132,000 Johnson & Johnson colleagues, who adjusted operational earnings growth* that passionately lead the way in improving the health exceeded the financial performance goals we and well-being of people around the world. set for the Company at the start of 2019. • We again made record investments in research and development (R&D)—more than $11 billion across our Pharmaceutical, Medical Devices Propelled by our people, products, and and Consumer businesses—as we maintained a purpose, we look forward to the future relentless pursuit of innovation to develop vital with great confidence and optimism scientific breakthroughs. as we remain committed to leading • We proudly launched new transformational across the spectrum of healthcare. medicines for untreated and treatment-resistant diseases, while gaining approvals for new uses of many of our medicines already in the market. Through proactive leadership across our enterprise, we navigated a constant surge • We deployed approximately $7 billion, of unique and complex challenges, spanning primarily in transactions that fortify our dynamic global issues, shifting political commitment to digital surgery for a more climates, industry and competitive headwinds, personalized and elevated standard of and an ongoing litigious environment. healthcare, and that enhance our position in consumer skin health. As we have experienced for 133 years, we • And our teams around the world continued can be sure that 2020 will present a new set of working to address pressing public health opportunities and challenges. -

Otc) Benefit Medication Formulary
OVER-THE-COUNTER (OTC) BENEFIT MEDICATION FORMULARY The OTC formulary is a list of medicines (Schedule 0-3) that will be paid from the OTC benefit, subject to the limit specified by the scheme. Metropolitan Generic Reference Price (METREF) will apply. Co-payments will apply for items amounting to more than the reference price. The formulary is subject to regular review. Metropolitan Health reserves the right to update and change the formulary when new information becomes available. IMPORTANT: Please note that the medication on this list is published on an annual basis. Medication and prices are subject to change based on new clinical information and/or pricing updates which may not be reflected on the list below. Product name Active Ingredient Strength Dosage form Schedule Nappi code Oral antiseptics and sore throat Andolex C Benzydamide SPR 1 710149 Andolex Oral Rinse Benzydamide . MOW 1 810576 Andolex Spray Benzydamide . SPR 1 816167 Betadine oral Povidone-iodine MOW 0 707937 Dermadine oral garg Povidone-iodine 1g/100ml MOW 0 799688 Iodised throat Antiseptics LOZ 1 892670 Iodised throat Antiseptics LOZ 1 810010 Medi-keel A Cetylpyridinium LOZ 1 741035 Medi-Keel A black currant Cetylpyridinium LOZ 1 884233 Medi-Keel A honey & lemon Cetylpyridinium LOZ 1 884225 Podine mouth wash Povidone-iodine MOW 0 785210 Septadine oral antiseptic Povidone-iodine MOW 0 819859 Steridine mouthwash and gargle liquid Povidone-iodine MOW 0 890365 Tcp antiseptic Antiseptics SLN 1 815950 Throflam Benzydamide MOW 1 712171 Throflam Benzydamine 22.5mg/15ml