Debridement of Chronic Wounds: a Systematic Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
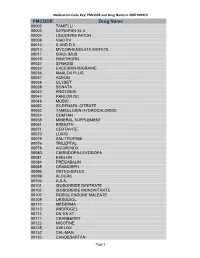
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS Cdc-Pdf
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00002 TAMIFLU 00003 DITROPAN XL II 00004 LIDODERM PATCH 00008 VIACTIV 00010 A AND D II 00013 MYCOPHENOLATE MOFETIL 00017 SIROLIMUS 00019 HAWTHORN 00027 SYNAGIS 00032 EXCEDRIN MIGRAINE 00036 MAALOX PLUS 00037 ACEON 00038 GLYSET 00039 SONATA 00042 PROTONIX 00044 PANLOR DC 00048 MOBIC 00052 SILDENAFIL CITRATE 00053 TAMSULOSIN HYDROCHLORIDE 00054 COMTAN 00058 MINERAL SUPPLEMENT 00061 BISMUTH 00071 CERTAVITE 00073 LUXIQ 00075 SAL-TROPINE 00076 TRILEPTAL 00078 AGGRENOX 00080 CARBIDOPA-LEVODOPA 00081 EXELON 00084 PREGABALIN 00085 ORAMORPH 00096 OSTEO-BIFLEX 00099 ALOCRIL 00100 A.S.A. 00101 ISOSORBIDE DINITRATE 00102 ISOSORBIDE MONONITRATE 00107 ROSIGLITAZONE MALEATE 00109 URSODIOL 00112 MEDERMA 00113 ANDROGEL 00114 DILTIA XT 00117 CRANBERRY 00123 NICOTINE 00125 AVELOX 00132 CAL-MAG 00133 CANDESARTAN Page 1 Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00148 PROLIXIN D 00149 D51/2 NS 00150 NICODERM CQ PATCH 00151 TUSSIN 00152 CEREZYME 00154 CHILDREN'S IBUPROFEN 00156 PROPOXACET-N 00159 KALETRA 00161 BISOPROLOL 00167 NOVOLIN N 00169 KETOROLAC TROMETHAMINE 00172 OPHTHALMIC OINTMENT 00173 ELA-MAX 00176 PREDNISOLONE ACETATE 00179 COLLOID SILVER 00184 KEPPRA 00187 OPHTHALMIC DROPS 00190 ABDEC 00191 HAPONAL 00192 SPECTRAVITE 00198 ENOXAPARIN SODIUM 00206 ACTONEL 00208 CELECOXIB 00209 GLUCOVANCE 00211 LEVALL 5.0 00213 PANTOPRAZOLE SODIUM 00217 TEMODAR 00218 CARBAMIDE PEROXIDE 00221 CHINESE HERBAL MEDS 00224 MILK AND MOLASSES ENEMA 00238 ZOLMITRIPTAN 00239 -

Advanced Textiles for Wound Care
Woodhead Publishing in Textiles: Number 85 Advanced textiles for wound care Edited by S. Rajendran Oxford Cambridge New Delhi © 2009 Woodhead Publishing Limited The Textile Institute and Woodhead Publishing The Textile Institute is a unique organisation in textiles, clothing and footwear. Incorporated in England by a Royal Charter granted in 1925, the Institute has individual and corporate members in over 90 countries. The aim of the Institute is to facilitate learning, recognise achievement, reward excellence and disseminate information within the global textiles, clothing and footwear industries. Historically, The Textile Institute has published books of interest to its members and the textile industry. To maintain this policy, the Institute has entered into partnership with Woodhead Publishing Limited to ensure that Institute members and the textile industry continue to have access to high calibre titles on textile science and technology. Most Woodhead titles on textiles are now published in collaboration with The Textile Institute. Through this arrangement, the Institute provides an Editorial Board which advises Woodhead on appropriate titles for future publication and suggests possible editors and authors for these books. Each book published under this arrangement carries the Institute’s logo. Woodhead books published in collaboration with The Textile Institute are offered to Textile Institute members at a substantial discount. These books, together with those published by The Textile Institute that are still in print, are offered on the Woodhead web site at: www.woodheadpublishing.com. Textile Institute books still in print are also available directly from the Institute’s website at: www.textileinstitutebooks.com. A list of Woodhead books on textile science and technology, most of which have been published in collaboration with The Textile Institute, can be found at the end of the contents pages. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

The Wound/Burn Guidelines –
doi: 10.1111/1346-8138.13288 Journal of Dermatology 2016; : 1–22 GUIDELINE The wound/burn guidelines – 6: Guidelines for the management of burns Yuichiro YOSHINO,1 Mikio OHTSUKA,2 Masakazu KAWAGUCHI,3 Keisuke SAKAI,4 Akira HASHIMOTO,5 Masahiro HAYASHI,3 Naoki MADOKORO,6 Yoshihide ASANO,7 Masatoshi ABE,8 Takayuki ISHII,9 Taiki ISEI,10 Takaaki ITO,11 Yuji INOUE,12 Shinichi IMAFUKU,13 Ryokichi IRISAWA,14 Masaki OHTSUKA,15 Fumihide OGAWA,16 Takafumi KADONO,7 Tamihiro KAWAKAMI,17 Ryuichi KUKINO,18 Takeshi KONO,19 Masanari KODERA,20 Masakazu TAKAHARA,21 Miki TANIOKA,22 Takeshi NAKANISHI,23 Yasuhiro NAKAMURA,24 Minoru HASEGAWA,9 Manabu FUJIMOTO,9 Hiroshi FUJIWARA,25 Takeo MAEKAWA,26 Koma MATSUO,27 Osamu YAMASAKI,15 Andres LE PAVOUX,28 Takao TACHIBANA,29 Hironobu IHN,12 The Wound/Burn Guidelines Committee 1Department of Dermatology, Japanese Red Cross Kumamoto Hospital, Kumamoto, 2Department of Dermatology, Fukushima Medical University, Fukushima, 3Department of Dermatology, Yamagata University Faculty of Medicine, Yamagata, 4Intensive Care Unit, Kumamoto University Hospital, Kumamoto, 5Department of Dermatology, Tohoku University Graduate School of Medicine, Miyagi, 6Department of Dermatology, Mazda Hospital, Hiroshima, 7Department of Dermatology, Faculty of Medicine,University of Tokyo, Tokyo, 8Department of Dermatology, Gunma University Graduate School of Medicine, Gunma, 9Department of Dermatology, Faculty of Medicine, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, Ishikawa, 10Department of Dermatology, Kansai -

Table 1B 2005 Community Prescription Numbers, Together with Government
TABLE 1B 2005 COMMUNITY PRESCRIPTION NUMBERS, TOGETHER WITH GOVERNMENT AND PATIENT COSTS FOR PBS-LISTED DRUGS Table 1B includes an estimate of community (non-public hospital) prescription numbers for the 2005 calendar year, costs (government and patient contribution) for the items with a four digit PBS/RPBS code, together with the defined daily dose (DDD) where assigned. There is no cost information available for items with a five digit Amfac drug code. Table 1 exclude the presentation of information on any item with an estimated community use of less than 110 prescriptions in 2005. The prescription items are arranged by ATC code on generic name, and by form and strength using either the PBS drug code (4 digit) or, for non-PBS items, the Amfac drug code (5 digit). Consult the index (page 255) by generic drug name to obtain the appropriate ATC code. An index by 2nd level of the ATC classification follows: ALIMENTARY TRACT AND METABOLISM PAGE NO A01 STOMATOLOGICAL PREPARATIONS 181 A02 DRUGS FOR ACID RELATED DISORDERS 182 A03 DRUGS FOR FUNCTIONAL GASTROINTESTINAL DISORDERS 185 A04 ANTIEMETICS AND ANTINAUSEANTS 187 A05 BILE AND LIVER THERAPY 188 A06 LAXATIVES 189 A07 ANTIDIARRHOEALS, INTESTINAL 191 ANTIINFLAMMATORY/ANTIINFECTIVE AGENTS A08 ANTIOBESITY PREPARATIONS, EXCLUDING DIET PRODUCTS 193 A09 DIGESTIVES, INCLUDING ENZYMES 194 A10 DRUGS USED IN DIABETES 195 A11 VITAMINS 197 A12 MINERAL SUPPLEMENTS 199 A14 ANABOLIC AGENTS FOR SYSTEMIC USE 200 177 BLOOD AND BLOOD FORMING ORGANS B01 ANTITHROMBOTIC AGENTS 201 B02 ANTIHAEMORRHAGICS 203 B03 -

Pharmacy Program and Drug Formulary
Pharmacy Program and Drug Formulary Secure Horizons Group Retiree Medicare Advantage Plan n Pharmacy Program Description n Platinum Plus Enhanced Formulary California Benefits Effective January 1, 2006 Table of Contents Your Secure Horizons Group Retiree Medicare Advantage Plan Prescription Drug Benefit........................................................................................................ iii Secure Horizons Pharmacy Program Definitions .................................................................... iii What Is the Platinum Plus Enhanced Formulary? ....................................................................iv Where to Have Your Prescriptions Filled .................................................................................iv Preferred and Non-Preferred Network Pharmacies .................................................................iv Network Preferred Pharmacy Locations ..................................................................................iv How to Fill a Prescription at a Network Pharmacy ...................................................................v Mail Service Pharmacy ..............................................................................................................v Secure Horizons Group Retiree Medicare Advantage Plan Offers a Two-Part Prescription Drug Benefit ..........................................................................vii Part 1 – Medicare Part D Prescription Drug Coverage ...................................................vii How Your Medicare -

Chronic Venous Ulcers: a Comparative Effectiveness Review of Treatment Modalities Comparative Effectiveness Review Number 127
Comparative Effectiveness Review Number 127 Chronic Venous Ulcers: A Comparative Effectiveness Review of Treatment Modalities Comparative Effectiveness Review Number 127 Chronic Venous Ulcers: A Comparative Effectiveness Review of Treatment Modalities Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Contract No. 290-2007-10061-I Prepared by: Johns Hopkins University Evidence-based Practice Center Baltimore, MD Investigators Jonathan Zenilman, M.D. M. Frances Valle, D.N.P., M.S. Mahmoud B. Malas, M.D., M.H.S. Nisa Maruthur, M.D., M.H.S. Umair Qazi, M.P.H. Yong Suh, M.B.A., M.Sc. Lisa M. Wilson, Sc.M. Elisabeth B. Haberl, B.A. Eric B. Bass, M.D., M.P.H. Gerald Lazarus, M.D. AHRQ Publication No. 13(14)-EHC121-EF December 2013 Erratum January 2014 This report is based on research conducted by the Johns Hopkins University Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 290-2007-10061-I). The findings and conclusions in this document are those of the author(s), who are responsible for its contents; the findings and conclusions do not necessarily represent the views of AHRQ. Therefore, no statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. The information in this report is intended to help health care decisionmakers—patients and clinicians, health system leaders, and policymakers, among others—make well-informed decisions and thereby improve the quality of health care services. -

The Effect of Iodine Based Products on Unicellular Algae from Genus Prototheca
The Effect of Iodine Based Products on Unicellular Algae from Genus Prototheca University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca, Faculty of Veterinary Medicine, 3-5Sorin Mănăştur RĂPUNTEAN Street, *, 400372, Gheorghe Romania RĂPUNTEAN, Flore CHIRILǍ, Nicodim Iosif FIŢ, George Cosmin NADǍŞ *Corresponding author: [email protected] Bulletin UASVM Veterinary Medicine 72(2) / 2015, Print ISSN 1843-5270; Electronic ISSN 1843-5378 DOI:10.15835/buasvmcn-vm: 11439 Abstract Iodine based products have been and are still used in medicine as disinfectant or antiseptic substances, because of their bactericidal, sporicidal, protocidal and disinfection effect. Algaecide effect has been lessPrototheca studied and represents a strong motivation for this study. This paper is aiming to test the sensitivityPrototheca to zopfii iodine based products of unicellular algae of the genus . Such products might be used for the treatment of diseases involving these pathogens. A total number of twenty-two strains isolated from cows with mastitis or bovine shelters, Protothecawere tested wickerhamii within this study. The algal strains were identified based on morphological characteristics (shape, size, presence of endospores), cultural (liquid and solid media characteristics) and biochemical (fermentation of sugars). ATCC 16529 reference strain was also included in the evaluation. Iodinated products used were represented by: Lugol solution, iodine tincture, betadine, videne and potassium iodide. Determination of the inhibitory effect was measured by diffusion technique in agarose gel and by liquid medium dilution method. For the tincture of iodine and betadine, the inhibitory effect was also appreciated in relation with the time of contact (5, 10, 15, 30 and 60 minutes). Prototheca zopfii By the agar diffusion technique, it was found that inhibition zones varying sizes were correlated with the composition of those products. -

Table 1 2009 Community Prescription Numbers
TABLE 1 2009 COMMUNITY PRESCRIPTION NUMBERS, TOGETHER WITH GOVERNMENT AND PATIENT COSTS FOR PBS LISTED DRUGS Table 1 includes an estimate of community prescription numbers for the 2009 calendar year, costs (government and patient contribution) for the items with a four digit PBS/RPBS code, together with the defined daily dose (DDD) where assigned. There is no cost information available for items with a five digit Amfac drug code. Table 1 excludes the presentation of information on any item with an estimated community use of less than 110 prescriptions in 2009. The prescription items are arranged by ATC code on generic name, and by form and strength using either the PBS drug code (4 digit plus alpha) or, for non-PBS items, the Amfac drug code (5 digit). Note that in this edition, “Item type” has been added to distinguish between PBS drug code and non-PBS drug code, for instance, P refers to PBS drug code and A refers to Amfac drug code. Note that Anatomical Therapeutic Chemical (ATC) classification index with Defined Daily Doses (DDDs) 2010 is used in all statistics published in this edition (refer to WHO collaborating Centre for Drug Statistics Methodology, ATC classification index with DDDs 2010). An index by second level of the ATC classification follows: ALIMENTARY TRACT AND METABOLISM 43 A01 STOMATOLOGICAL PREPARATIONS 43 A02 DRUGS FOR ACID RELATED DISORDERS 44 A03 DRUGS FOR FUNCTIONAL GASTROINTESTINAL DISORDERS 47 A04 ANTIEMETICS AND ANTINAUSEANTS 48 A05 BILE AND LIVER THERAPY 49 A06 LAXATIVES 51 A07 ANTIDIARRHOEALS, INTESTINAL ANTIINFLAMMATORY/ANTIINFECTIVES -

A Comprehensive Review of Topical Odor-Controlling Treatment Options for Chronic Wounds
Wound Care J Wound Ostomy Continence Nurs. 2016;43(6):598-609. Published by Lippincott Williams & Wilkins A Comprehensive Review of Topical Odor-Controlling 3 Treatment Options for Chronic Wounds Alma Akhmetova ¿ Timur Saliev ¿ Iain U. Allan ¿ Matthew J. Illsley ¿ Talgat Nurgozhin ¿ Sergey Mikhalovsky ABSTRACT The process of wound healing is often accompanied by bacterial infection or critical colonization, resulting in protracted infl ammation, delayed reepithelization, and production of pungent odors. The malodor produced by these wounds may lower health-related quality of life and produce psychological discomfort and social isolation. Current management focuses on reducing bacterial activity within the wound site and absorbing malodorous gases. For example, charcoal-based materials have been incorporated into dressing for direct adsorption of the responsible gases. In addition, multiple topical agents, including silver, iodine, honey, sugar, and essential oils, have been suggested for incorporation into dressings in an attempt to control the underlying bacterial infection. This review describes options for controlling malodor in chronic wounds, the benefi ts and drawbacks of each topical agent, and their mode of action. We also discuss the use of subjective odor evaluation techniques to assess the effi cacy of odor-controlling therapies. The perspectives of employing novel biomaterials and technologies for wound odor management are also presented. KEY WORDS: Chronic wound , Control , Dressing , Infection , Malodor , Odor , Therapy . INTRODUCTION evidence concerning the effi cacy of various interventions de- signed to reduce malodor produced by some chronic wounds Common clinical manifestations associated with wounds in- remains sparse. 5 clude pain, itch, odor, bleeding, and production of exudate; Unpleasant odor is often found in chronic wounds, and however, malodor is recognized as one of most distressful especially those that have not closed following 3 months of aspects of some wounds. -

Antimicrobial Dressing for Diabetic Foot Ulcer Colonized with MRSA
OnLine Journal of Biological Sciences Review Antimicrobial Dressing for Diabetic Foot Ulcer Colonized with MRSA Parthasarathy Ravichandran and Sai Prasad Chitti Department of Biochemistry and Molecular Biology, Pondicherry University, Pondicherry, India Article history Abstract: Wound healing in patients with diabetic foot ulcer differs Received: 24-05-2015 among people. The wound healing process was influenced by factors like Revised: 26-07-2015 nature of the wound, tissue and an immunity of a person. Any measure Accepted: 02-11-2015 taken to control bacterial colonies in wound plays a significant role in wound healing. However, recent emergence of Methicillin-resistant Corresponding Author: Parthasarathy Ravichandran Staphylococcus Aureus (MRSA) associated with chronic wounds created Department of Biochemistry health concerns worldwide. An MRSA colony present in diabetic wounds and Molecular Biology, vulnerable to prolong the wound healing has reported worldwide. Since Pondicherry University, MRSA are resistant to a wide range of antibiotics, choosing appropriate Pondicherry, India dressings to treat MRSA colonized wounds has become a challenge. Email: [email protected] Either synthetic or natural antimicrobial agents are used to develop dressings that combat against MRSA infections. In today’s practice, the incidence of chronic wounds and its associated socioeconomic consequences is rising despite effort and advances in wound management. In this review, an attempt made to summarize various antimicrobial dressings based on its activity against MRSA. Keywords: Wound, Honey, Silver, Antimicrobial, MRSA Introduction derivatives, including Methicillin are used for the treatments of infections caused by Staphylococcus The estimated prevalence of diabetes covers 382 aureus (Rayner and Munckhof, 2005). The misuse and million people (Tao et al ., 2015). -

“Furuta Method” for Effective Pressure Ulcer Treatment: a Retrospective Study Katsunori Furuta1,2*, Fumihiro Mizokami2, Hitoshi Sasaki3 and Masato Yasuhara4
Furuta et al. Journal of Pharmaceutical Health Care and Sciences (2015) 1:21 DOI 10.1186/s40780-015-0021-8 RESEARCH Open Access Active topical therapy by “Furuta method” for effective pressure ulcer treatment: a retrospective study Katsunori Furuta1,2*, Fumihiro Mizokami2, Hitoshi Sasaki3 and Masato Yasuhara4 Abstract Background: We newly proposed that “Furuta method,” a pharmacist intervention guidelines, is a topical ointment therapy that considers the physical properties and moist environment of wounds for pressure ulcer (PU) treatment. The aim of this multicenter retrospective study was to investigate the effectiveness of this method for PU. Methods: A total of 888 consecutive patients who underwent treatment for PU at 37 hospitals and five dispensing pharmacies in Japan between August 2010 and July 2014 were included in the study. Based on a survey on compliance to “Furuta method,” single-blind allocation was conducted into compliance (n = 437) and non-compliance (n = 451) groups, followed by a retrospective data collection. The primary and secondary outcomes were the healing period and rates of unhealed wounds, respectively. Data was expressed as mean ± standard deviation. Two-sided log rank tests were used for between-group comparisons of PU progression, whereas Kaplan–Meier plots were used for comparison between groups. We performed rigorous adjustment for marked differences in baseline patient characteristics by propensity score (PS) matching. Results: After PS matching, patients were categorized as DESIGN-R d2 (n = 202), D3 (n = 130), D4 and 5 (n = 76), and DU (n = 76). In terms of the healing period, the patients in the compliance groups healed faster than those in the non-compliance groups in d2 (23.6 ± 36.8 vs.