California Health & Wellness
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Scabies in Healthcare Facilities
Scabies in Healthcare Facilities Tammra L. Morrison, RN BSN Healthcare Associated Infections Coordinator Communicable Disease Branch, Epidemiology Section December 9, 2016 Symptoms • In a person who has never had scabies: • May take 4-6 weeks for symptom onset • In a person who has had scabies in the past: • Symptoms may start in 1-4 days • May be spread PRIOR to symptom onset What to Look for • Intense itching • Especially at night • Pimple-like itchy rash • May affect entire body OR Dermatologie.md common sites: • Wrist, elbow, armpit, webbing between the fingers, nipple, penis, waist, belt-line, and buttocks • Burrows (tunnels) may be seen on the skin • Tiny raised and crooked grayish-white or skin- colored lines Transmission • Direct, prolonged, skin-to-skin contact with an infested person • Sexual partners • Household members • Quick handshake/hug will usually not spread scabies How Long Do Mites Live? • 1-2 months on a person • 48-72 hours off a person • Scabies mites will die at 122 degrees for 10 minutes Webmd.com 5 Diagnosis • Customary appearance and distribution of the rash and presence of burrows. • Confirm diagnosis: • Obtain a skin scraping to examine under a microscope for mites, eggs, or mite fecal matter • Person can still be infested even if mites, eggs, or fecal matter cannot be found • Typically fewer than 10-15 mites present on the entire body • **Crusted scabies may be thousands of mites and should be considered highly contagious** How Do You Treat Scabies? 7 Treatment • Available only by prescription • No "over-the-counter“ -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

CCA Senior Care Options Formulary
Commonwealth Care Alliance Senior Care Option HMO SNP 2021 List of Covered Drugs Formulary 30 Winter Street • Boston, MA 02108 PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN This formulary was updated on 08/01/2021. For more recent information or other questions, please contact Senior Care Options Program (HMO SNP) Member Services, at 1-866-610-2273 or, for TTY users, 711, 8 a.m. – 8 p.m., 7 days a week, or visit www.commonwealthcaresco.org. HPMS Approved Formulary File Submission ID 00021589, Version Number 13 Senior Care Options Program (HMO SNP) 2021 Formulary (List of Covered Drugs) PLEASE READ: THIS DO CUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN HPMS Approved Formulary File Submission ID 00021589, Version Number 13 Note to existing members: This formulary has changed since last year. Please review this document to make sure that it still contains the drugs you take. When this drug list (formulary) refers to “we,” “us”, or “our,” it means Commonwealth Care Alliance. When it refers to “plan” or “our plan,” it means 2021 Senior Care Options Program. This document includes list of the drugs (formulary) for our plan which is current as of 08/01/2021. This formulary document applies to all SCO members. For an updated formulary, please contact us. Our contact information, along with the date we last updated the formulary, appears on the front and back cover pages. You must generally use network pharmacies to use your prescription drug benefit. Benefits, formulary, pharmacy n etwork, and/or copayments/coinsurance may change on January 1, 2022, and from time to time during the year. -

Triclosan Disrupts Thyroid Hormones: Mode-Of-Action, Developmental Susceptibility, and Determination of Human Relevance
Triclosan Disrupts Thyroid Hormones: Mode-of-Action, Developmental Susceptibility, and Determination of Human Relevance Katie Beth Paul “A dissertation submitted to the faculty of the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Curriculum of Toxicology.” Chapel Hill 2011 Approved by: Kim L. R. Brouwer, Pharm.D., Ph.D. Kevin M. Crofton, Ph.D. Michael J. DeVito, Ph.D. Philip C. Smith, Ph.D James A. Swenberg, D.V.M., Ph.D. ©2011 Katie Beth Paul ALL RIGHTS RESERVED ii Abstract Katie Beth Paul Triclosan Disrupts Thyroid Hormones: Mode-of-Action, Developmental Susceptibility, and Determination of Human Relevance (Under the direction of Kevin M. Crofton, Ph.D.) Preliminary study demonstrated that triclosan (TCS), a bacteriostat in myriad consumer products, decreases serum thyroxine (T4) in rats. Adverse neurodevelopmental consequences result from thyroid hormone (TH) disruption; therefore determination of whether TCS disrupts THs during development, its mode-of-action (MOA), and the human relevance is critical. This research tested the hypothesis that TCS disrupts THs via activation of pregnane X and constitutive androstane receptors (PXR, CAR), mediating Phase I-II enzyme and hepatic transporter expression and protein changes, thereby increasing catabolism and elimination of THs, resulting in decreased TH concentrations. For Aim One, the hypothesized MOA was assessed using weanling female Long-Evans rats orally exposed to TCS (0-1000 mg/kg/day) for four days. Serum T4 decreased 35% at 300 mg/kg/day. Activity and expression of markers of Phase I (Cyp2b, Cyp3a1) and Phase II (Ugt1a1, Sult1c1) metabolism were moderately induced, consistent with PXR and/or CAR activation and increased hepatic catabolism. -

Medicines That Affect Fluid Balance in the Body
the bulk of stools by getting them to retain liquid, which encourages the Medicines that affect fluid bowels to push them out. balance in the body Osmotic laxatives e.g. Lactulose, Macrogol - these soften stools by increasing the amount of water released into the bowels, making them easier to pass. Older people are at higher risk of dehydration due to body changes in the ageing process. The risk of dehydration can be increased further when Stimulant laxatives e.g. Senna, Bisacodyl - these stimulate the bowels elderly patients are prescribed medicines for chronic conditions due to old speeding up bowel movements and so less water is absorbed from the age. stool as it passes through the bowels. Some medicines can affect fluid balance in the body and this may result in more water being lost through the kidneys as urine. Stool softener laxatives e.g. Docusate - These can cause more water to The medicines that can increase risk of dehydration are be reabsorbed from the bowel, making the stools softer. listed below. ANTACIDS Antacids are also known to cause dehydration because of the moisture DIURETICS they require when being absorbed by your body. Drinking plenty of water Diuretics are sometimes called 'water tablets' because they can cause you can reduce the dry mouth, stomach cramps and dry skin that is sometimes to pass more urine than usual. They work on the kidneys by increasing the associated with antacids. amount of salt and water that comes out through the urine. Diuretics are often prescribed for heart failure patients and sometimes for patients with The major side effect of antacids containing magnesium is diarrhoea and high blood pressure. -

Medicare Modernization Act Final Guidelines
MEDICARE MODERNIZATION ACT FINAL GUIDELINES -- FORMULARIES CMS Strategy for Affordable Access to Comprehensive Drug Coverage Guidelines for Reviewing Prescription Drug Plan Formularies and Procedures 1. Purpose of the Guidance This paper is final guidance on how CMS will review Medicare prescription drug benefit plans to assure that beneficiaries receive clinically appropriate medications at the lowest possible cost. Two key requirements in the Medicare Modernization Act (MMA) are to assure that drug plans provide access to medically necessary treatments for all and do not discriminate against any particular types of beneficiaries, and to encourage and support the use of approaches to drug benefit management that are proven and in widespread use in prescription drug plans today. The goal is for plans to provide high-quality cost-effective drug benefits by negotiating the best possible prices and using effective drug utilization management techniques. This goal can be achieved through a CMS drug benefit review strategy that facilitates appropriate beneficiary access to all medically necessary Part D covered drugs along with plan flexibility to develop efficient benefit designs, thus bringing drug benefit strategies that are already providing effective coverage to millions of seniors and people with a disability to the Medicare population. Our formulary review process focuses on three areas: 1. Pharmacy and Therapeutics (P&T) committees. CMS will require P&T committees to rely on widely-used best practices, reinforced by MMA standards. CMS oversight of these processes will assure that plan formularies are designed to provide appropriate, up-to-date access for beneficiaries and give plans the flexibility to offer benefit designs that provide affordable access to medically necessary drugs. -

Pharmaceuticals and Endocrine Active Chemicals in Minnesota Lakes
Pharmaceuticals and Endocrine Active Chemicals in Minnesota Lakes May 2013 Authors Mark Ferrey Contributors/acknowledgements The MPCA is reducing printing and mailing costs This report contains the results of a study that by using the Internet to distribute reports and characterizes the presence of unregulated information to wider audience. Visit our website contaminants in Minnesota’s lakes. The study for more information. was made possible through funding by the MPCA reports are printed on 100 percent post- Minnesota Clean Water Fund and by funding by consumer recycled content paper manufactured the U.S. Environmental Protection Agency without chlorine or chlorine derivatives. (EPA), which facilitated the sampling of lakes for this study. The Minnesota Pollution Control Agency (MPCA) thanks the following for assistance and advice in designing and carrying out this study: Steve Heiskary, Pam Anderson, Dereck Richter, Lee Engel, Amy Garcia, Will Long, Jesse Anderson, Ben Larson, and Kelly O’Hara for the long hours of sampling for this study. Cynthia Tomey, Kirsten Anderson, and Richard Grace of Axys Analytical Labs for the expert help in developing the list of analytes for this study and logistics to make it a success. Minnesota Pollution Control Agency 520 Lafayette Road North | Saint Paul, MN 55155-4194 | www.pca.state.mn.us | 651-296-6300 Toll free 800-657-3864 | TTY 651-282-5332 This report is available in alternative formats upon request, and online at www.pca.state.mn.us. Document number: tdr-g1-16 Contents Contents ........................................................................................................................................... -

Toxicological Profile for Phenol
PHENOL 21 3. HEALTH EFFECTS 3.1 INTRODUCTION The primary purpose of this chapter is to provide public health officials, physicians, toxicologists, and other interested individuals and groups with an overall perspective on the toxicology of phenol. It contains descriptions and evaluations of toxicological studies and epidemiological investigations and provides conclusions, where possible, on the relevance of toxicity and toxicokinetic data to public health. It should be noted that phenol is the simplest form, or parent compound, of the class of chemicals commonly referred to as phenols or phenolics, many of which are natural substances widely distributed throughout the environment. There is some confusion in the literature as to the use of the term ‘phenol’; in some cases, it has been used to refer to a particular phenolic compound that is more highly substituted than the parent compound (Doan et al. 1979), whereas in other cases, it has been used to refer to the class of phenolic compounds (Beveridge 1997). This chapter, however, addresses only those health effects that can be directly attributable to the parent compound, monohydroxybenzene, or phenol. As Deichmann and Keplinger (1981) note: “It cannot be overemphasized that the structure-activity relationships of phenol and phenol derivatives vary widely, and that to accept the properties of individual phenolic compounds as being those of phenol is a misconception and leads to error and confusion.” A glossary and list of acronyms, abbreviations, and symbols can be found in Appendix C at the end of this profile. 3.2 DISCUSSION OF HEALTH EFFECTS BY ROUTE OF EXPOSURE To help public health professionals and others address the needs of persons living or working near hazardous waste sites, the information in this section is organized first by route of exposure (inhalation, oral, and dermal) and then by health effect (death, systemic, immunological, neurological, reproductive, developmental, genotoxic, and carcinogenic effects). -

Prescription Drug Benefit Administration Drug Formulary
SECTION 8 PHARMACY Prescription Drug Benefit Administration Health Alliance administers pharmacy benefits in conjunction with OptumRx, a pharmacy benefit management (PBM) company. This function is coordinated by the Pharmacy Department at Health Alliance. Activities of this department include: Pharmacy network development and maintenance Third-party claims processor relations, contract development and management Manufacturer discount contracting Pharmacy and Therapeutics Committee (P&T) support Drug formulary coordination and management Utilization Management Department clinical support Medical Directors Committee and administrative support Quality Improvement Committee support Assistance in improving quality measures related to medications Pharmacy utilization reporting and physician support Customer Service and Claims Departments support Medicare Part D Formulary coordination and management Drug Formulary The Health Alliance drug formularies were created to assist in the management of ever-increasing costs of prescription medications. The use of formularies to provide physicians with a reference for cost-effective medical treatment has been used successfully in health insurance organizations throughout the country. Formularies were created under the guidance of physicians and pharmacists representing most specialties. The P&T Committee evaluates the need of patients, use of products and cost-effectiveness as factors to determine the formulary choices. In all cases, available bioequivalence data and therapeutic activity are considered. The P&T Committee meets on a regular basis to evaluate the changing needs of physicians and patients. We urge you to provide recommendations for improvement of the drug formularies. It is our belief that the drug formularies can enhance your ability to provide quality, cost-effective care to your Health Alliance patients. The use of generic and over-the-counter (OTC) products is highly recommended when applicable. -

The Effects of Combination Treatments on Drug Resistance in Chronic Myeloid Leukaemia: an Evaluation of the Tyrosine Kinase Inhibitors Axitinib and Asciminib H
Lindström and Friedman BMC Cancer (2020) 20:397 https://doi.org/10.1186/s12885-020-06782-9 RESEARCH ARTICLE Open Access The effects of combination treatments on drug resistance in chronic myeloid leukaemia: an evaluation of the tyrosine kinase inhibitors axitinib and asciminib H. Jonathan G. Lindström and Ran Friedman* Abstract Background: Chronic myeloid leukaemia is in principle a treatable malignancy but drug resistance is lowering survival. Recent drug discoveries have opened up new options for drug combinations, which is a concept used in other areas for preventing drug resistance. Two of these are (I) Axitinib, which inhibits the T315I mutation of BCR-ABL1, a main source of drug resistance, and (II) Asciminib, which has been developed as an allosteric BCR-ABL1 inhibitor, targeting an entirely different binding site, and as such does not compete for binding with other drugs. These drugs offer new treatment options. Methods: We measured the proliferation of KCL-22 cells exposed to imatinib–dasatinib, imatinib–asciminib and dasatinib–asciminib combinations and calculated combination index graphs for each case. Moreover, using the median–effect equation we calculated how much axitinib can reduce the growth advantage of T315I mutant clones in combination with available drugs. In addition, we calculated how much the total drug burden could be reduced by combinations using asciminib and other drugs, and evaluated which mutations such combinations might be sensitive to. Results: Asciminib had synergistic interactions with imatinib or dasatinib in KCL-22 cells at high degrees of inhibition. Interestingly, some antagonism between asciminib and the other drugs was present at lower degrees on inhibition. -
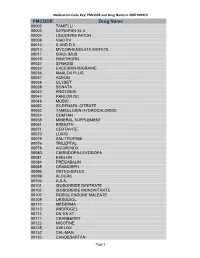
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS Cdc-Pdf
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00002 TAMIFLU 00003 DITROPAN XL II 00004 LIDODERM PATCH 00008 VIACTIV 00010 A AND D II 00013 MYCOPHENOLATE MOFETIL 00017 SIROLIMUS 00019 HAWTHORN 00027 SYNAGIS 00032 EXCEDRIN MIGRAINE 00036 MAALOX PLUS 00037 ACEON 00038 GLYSET 00039 SONATA 00042 PROTONIX 00044 PANLOR DC 00048 MOBIC 00052 SILDENAFIL CITRATE 00053 TAMSULOSIN HYDROCHLORIDE 00054 COMTAN 00058 MINERAL SUPPLEMENT 00061 BISMUTH 00071 CERTAVITE 00073 LUXIQ 00075 SAL-TROPINE 00076 TRILEPTAL 00078 AGGRENOX 00080 CARBIDOPA-LEVODOPA 00081 EXELON 00084 PREGABALIN 00085 ORAMORPH 00096 OSTEO-BIFLEX 00099 ALOCRIL 00100 A.S.A. 00101 ISOSORBIDE DINITRATE 00102 ISOSORBIDE MONONITRATE 00107 ROSIGLITAZONE MALEATE 00109 URSODIOL 00112 MEDERMA 00113 ANDROGEL 00114 DILTIA XT 00117 CRANBERRY 00123 NICOTINE 00125 AVELOX 00132 CAL-MAG 00133 CANDESARTAN Page 1 Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00148 PROLIXIN D 00149 D51/2 NS 00150 NICODERM CQ PATCH 00151 TUSSIN 00152 CEREZYME 00154 CHILDREN'S IBUPROFEN 00156 PROPOXACET-N 00159 KALETRA 00161 BISOPROLOL 00167 NOVOLIN N 00169 KETOROLAC TROMETHAMINE 00172 OPHTHALMIC OINTMENT 00173 ELA-MAX 00176 PREDNISOLONE ACETATE 00179 COLLOID SILVER 00184 KEPPRA 00187 OPHTHALMIC DROPS 00190 ABDEC 00191 HAPONAL 00192 SPECTRAVITE 00198 ENOXAPARIN SODIUM 00206 ACTONEL 00208 CELECOXIB 00209 GLUCOVANCE 00211 LEVALL 5.0 00213 PANTOPRAZOLE SODIUM 00217 TEMODAR 00218 CARBAMIDE PEROXIDE 00221 CHINESE HERBAL MEDS 00224 MILK AND MOLASSES ENEMA 00238 ZOLMITRIPTAN 00239 -

Acqueous Parachlorophenol: Its Toxicity and Antimicrobial Effectiveness
Loyola University Chicago Loyola eCommons Master's Theses Theses and Dissertations 1969 Acqueous Parachlorophenol: Its Toxicity and Antimicrobial Effectiveness John W. Harrison Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_theses Part of the Medicine and Health Sciences Commons Recommended Citation Harrison, John W., "Acqueous Parachlorophenol: Its Toxicity and Antimicrobial Effectiveness" (1969). Master's Theses. 2367. https://ecommons.luc.edu/luc_theses/2367 This Thesis is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Master's Theses by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1969 John W. Harrison ACQUEOUS PAHACB.lil')HOPHENOL: I'I'S TOXICITY AND AN'I'IMICROBIAL EFFECTIVENESS by John Wylie Harrison, B.&., D.M.D. A Thesis Submitted to the Faculty of the Graduate School of Loyola University in Partial Fulfillment of the Requirements for the Degree of Master of Science June 1969 lftrary · · Loyora University Medical <:enbl Acknowledgements There are many people who deserve thanks for their assistance in the myriad of problems which inevitably arise in a research project of this na ture. I can only hope that my sincere gratitude for their efforts has been made personally evident. I am particularly indebted to B. Franklin Gurney, B.S., M.s •• D.D.S., F.A.C.D., for suggesting certain ideas which eventually led to the choice of this par ticular problem as a research project; and to Norman K.