Advanced Textiles for Wound Care
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mosby's Comprehensive Review of Radiography
PERPUSTAKAAN PRIBADI AN-NUR YOU’VE JUST PURCHASED MORE THAN A TEXTBOOK!* Evolve Student Resources for Callaway: Mosby’s Comprehensive Review of Radiography: The Complete Study Guide and Career Planner, Seventh Edition, include the following: • Radiography Practice Exams • Flashcards • Sample Resumes and Cover Letters Activate the complete learning experience that comes with each NEW textbook purchase by registering with your scratch-off access code at http://evolve.elsevier.com/Callaway/radiography/ If you purchased a used book and the scratch-off code at right has already been revealed, the code may have been used and cannot be re-used for registration. To purchase a new code to access these valuable study resources, simply follow the link above. Place Peel Off Sticker Here REGISTER TODAY! You can now purchase Elsevier products on Evolve! Go to evolve.elsevier.com/html/shop-promo.html to search and browse for products. * Evolve Student Resources are provided free with each NEW book purchase only. Mosby’s Comprehensive Review of Radiography The Complete Study Guide and Career Planner Seventh Edition This page intentionally left blank Mosby’s Comprehensive Review of Radiography The Complete Study Guide and Career Planner Seventh Edition William J. Callaway, MA, RT(R) Radiography Educator, Author, Speaker Springfield, Illinois 3251 Riverport Lane St. Louis, Missouri 63043 MOSBY’S COMPREHENSIVE REVIEW OF RADIOGRAPHY: THE COMPLETE STUDY GUIDE AND CAREER PLANNER, SEVENTH EDITION ISBN: 978-0-323-35423-3 Copyright © 2017 Elsevier Inc. All Rights Reserved. Previous editions copyrighted 2013, 2008, 2006, 2002, 2000, 1998, 1995. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. -

2017 Elsevier Foundation Annual Report
2017 Elsevier Foundation Report Médecins Sans Frontiers doctors conduct a Phase III rotavirus vaccine trial at Epicentre’s Niger Research Center at the Maradi Hospital, which receives an annual capacity building grant of $100,000 from the Elsevier Foundation. © KRISHAN Cheyenne/MSF White City students at a recent Imperial College London workshop with the student drone society. The Elsevier Foundation supported Maker’s Challenge space will launch in September, hosting future White City students as they navigate 3D printers, tackle robotics and many other tech challenges. © Imperial College London 2017 OWSD-Elsevier Foundation woman scientist award winner Felycia Edi Soetaredjo, PhD, Lecturer at Widya Mandala Surabaya Catholic University in Surabaya, Indonesia, was recognized for her work utilizing waste and cheap materials for environmental remediation of renewable energy. © Widya Mandala Surabaya Catholic University The Elsevier Foundation 2017 Board Report 2 Contents Foreword: Youngsuk “YS” Chi, President of the Elsevier Foundation I. The Elsevier Foundation 1. Who we are 2. Our Board 3. Our Programs 4. Our Future II. Our Programs 1. Health & Innovation 2. Research in Developing Countries 3. Diversity in STM 4. Technology for Development IV. Matching Gift V. Media Outreach VI. Financial Overview VII. Appendix The Elsevier Foundation 2017 Board Report 3 The Elsevier Foundation 2017 Board Report 4 Foreword On the occasion of the September 2017 Elsevier Foundation Board Meeting It has been an incredible privilege to steer collaboration in Sustainability. The TWAS the Elsevier Foundation’s development Elsevier Foundation Program provides over the last decade. I continue to be travel grants for PhD’s and postdoc inspired by the dedication and resolve of scientists studying in sustainability fields this group as we strive to tackle important and hosts case study competitions to global challenges. -
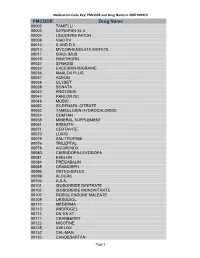
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS Cdc-Pdf
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00002 TAMIFLU 00003 DITROPAN XL II 00004 LIDODERM PATCH 00008 VIACTIV 00010 A AND D II 00013 MYCOPHENOLATE MOFETIL 00017 SIROLIMUS 00019 HAWTHORN 00027 SYNAGIS 00032 EXCEDRIN MIGRAINE 00036 MAALOX PLUS 00037 ACEON 00038 GLYSET 00039 SONATA 00042 PROTONIX 00044 PANLOR DC 00048 MOBIC 00052 SILDENAFIL CITRATE 00053 TAMSULOSIN HYDROCHLORIDE 00054 COMTAN 00058 MINERAL SUPPLEMENT 00061 BISMUTH 00071 CERTAVITE 00073 LUXIQ 00075 SAL-TROPINE 00076 TRILEPTAL 00078 AGGRENOX 00080 CARBIDOPA-LEVODOPA 00081 EXELON 00084 PREGABALIN 00085 ORAMORPH 00096 OSTEO-BIFLEX 00099 ALOCRIL 00100 A.S.A. 00101 ISOSORBIDE DINITRATE 00102 ISOSORBIDE MONONITRATE 00107 ROSIGLITAZONE MALEATE 00109 URSODIOL 00112 MEDERMA 00113 ANDROGEL 00114 DILTIA XT 00117 CRANBERRY 00123 NICOTINE 00125 AVELOX 00132 CAL-MAG 00133 CANDESARTAN Page 1 Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00148 PROLIXIN D 00149 D51/2 NS 00150 NICODERM CQ PATCH 00151 TUSSIN 00152 CEREZYME 00154 CHILDREN'S IBUPROFEN 00156 PROPOXACET-N 00159 KALETRA 00161 BISOPROLOL 00167 NOVOLIN N 00169 KETOROLAC TROMETHAMINE 00172 OPHTHALMIC OINTMENT 00173 ELA-MAX 00176 PREDNISOLONE ACETATE 00179 COLLOID SILVER 00184 KEPPRA 00187 OPHTHALMIC DROPS 00190 ABDEC 00191 HAPONAL 00192 SPECTRAVITE 00198 ENOXAPARIN SODIUM 00206 ACTONEL 00208 CELECOXIB 00209 GLUCOVANCE 00211 LEVALL 5.0 00213 PANTOPRAZOLE SODIUM 00217 TEMODAR 00218 CARBAMIDE PEROXIDE 00221 CHINESE HERBAL MEDS 00224 MILK AND MOLASSES ENEMA 00238 ZOLMITRIPTAN 00239 -

Alternative Textbooks Publishers
ALTERNATIVE TEXT PUBLISHERS TUTORING SERVICES 2071 CEDAR HALL ALTERNATIVE TEXT PUBLISHERS Below is a list of all the publishers we work with to provide alternative text files. Aaronco Pet Products, Inc. Iowa State: Extension and Outreach Abrams Publishing Jones & Bartlett Learning ACR Publications KendallHunt Publishing Alpine Publisher Kogan Page American Health Information Management Associations Labyrinth Learning American Hotels and Lodging Legal Books Distributing American Technical Publishers Lippincott Williams and Wilkins American Welding Society Longleaf Services AOTA Press Lynne Rienner Publishers Apress Macmillan Higher Education Associated Press Manning Publications ATI Nursing Education McGraw-Hill Education American Water Works Association Mike Holt Enterprises Baker Publishing Group Morton Publishing Company Barron's Mosby Bedford/St. Martin's Murach Books Bison Books NAEYC Blackwell Books NASW Press National Board for Certification in Bloomsbury Publishing Dental Laboratory Technology (NBC) National Restaurant Association/ Blue Book, The ServSafe Blue Door Publishing Office of Water Programs BookLand Press Openstax Broadview Press O'Reilly Media Building Performance Institute, Inc. Oxford University Press BVT Publishing Paradigm Publishing Cadquest Pearson Custom Editions ALTERNATIVE TEXT PUBLISHERS Cambridge University Press Pearson Education CE Publishing Peguin Books Cengage Learning Pennwell Books Charles C. Thomas, Publisher Picador Charles Thomas Publisher Pioneer Drama Cheng & Tsui PlanningShop Chicago Distribution -

Synthesizing Qualitative Evidence
Synthesizing Qualitative Evidence Alan Pearson Suzi Robertson-Malt Leslie Rittenmeyer JBI-Book2 Cov.indd 1 9/8/11 7:54 PM P1: OSO/OVY P2: OSO/OVY QC: OSO/OVY T1: OSO LWBK991-Book2-FM LWBK991-Sample-v1 September 1, 2011 7:20 Synthesizing Qualitative Evidence Alan Pearson Suzi Robertson-Malt Leslie Rittenmeyer Lippincott-Joanna Briggs Institute Synthesis Science in Healthcare Series: Book 2 1 P1: OSO/OVY P2: OSO/OVY QC: OSO/OVY T1: OSO LWBK991-Book2-FM LWBK991-Sample-v1 September 1, 2011 7:20 2 Publisher: Anne Dabrow Woods, MSN, RN, CRNP, ANP-BC Editor: Professor Alan Pearson, AM, Professor of Evidence Based Healthcare and Executive Director of the Joanna Briggs Institute; Faculty of Health Sciences at the University of Adelaide, SA 5005 Australia Production Director: Leslie Caruso Managing Editor, Production: Erika Fedell Production Editor: Sarah Lemore Creative Director: Larry Pezzato Copyright C 2011 Lippincott Williams & Wilkins, a Wolters Kluwer business. Two Commerce Square 2001 Market Street Philadelphia, PA 19103 ISBN 978-1-4511-6384-1 Printed in Australia All rights reserved. This book is protected by copyright. No part of this book may be reproduced or transmitted in any form or by any means, including as photocopies or scanned-in or other electronic copies, or utilized by any information storage and retrieval system without written permission from the copyright owner, except for brief quotations embodied in critical articles and reviews. Materials appearing in this book prepared by individuals as part of their official duties as U.S. government employees are not covered by the above mentioned copyright. -

Debridement of Chronic Wounds: a Systematic Review
Health Technology Assessment 1999; Vol. 3: No. 17 (Pt 1) Review The debridement of chronic wounds: a systematic review M Bradley N Cullum T Sheldon Health Technology Assessment NHS R&D HTA Programme HTA HTA How to obtain copies of this and other HTA Programme reports. An electronic version of this publication, in Adobe Acrobat format, is available for downloading free of charge for personal use from the HTA website (http://www.hta.ac.uk). A fully searchable CD-ROM is also available (see below). Printed copies of HTA monographs cost £20 each (post and packing free in the UK) to both public and private sector purchasers from our Despatch Agents. Non-UK purchasers will have to pay a small fee for post and packing. For European countries the cost is £2 per monograph and for the rest of the world £3 per monograph. You can order HTA monographs from our Despatch Agents: – fax (with credit card or official purchase order) – post (with credit card or official purchase order or cheque) – phone during office hours (credit card only). Additionally the HTA website allows you either to pay securely by credit card or to print out your order and then post or fax it. Contact details are as follows: HTA Despatch Email: [email protected] c/o Direct Mail Works Ltd Tel: 02392 492 000 4 Oakwood Business Centre Fax: 02392 478 555 Downley, HAVANT PO9 2NP, UK Fax from outside the UK: +44 2392 478 555 NHS libraries can subscribe free of charge. Public libraries can subscribe at a very reduced cost of £100 for each volume (normally comprising 30–40 titles). -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

REQUIRED BOOKS WILL BE USED THROUGHOUT CURRICULUM-DO NOT RENT OR SELL JUNIOR I Textbook List Updated 3/29/2017
SAM HOUSTON STATE UNIVERSITY School of Nursing Fall 2017 JUNIOR I Textbook List Updated 7/5/2017 ALL BOOKS ARE REQUIRED UNLESS OTHERWISE NOTED Your books may be purchased through the campus Barns & Noble Bookstore (936) 294-1864. Mosby/Elsevier textbooks will package their books. The package is available as E-book only or Print and E-book. Books noted as ‘optional’ are not included in the Mosby/Elsevier textbook package. The publishers not included in the Mosby/Elsevier textbook package are highlighted for your convenience. NURS 3410 – Health Assessment 1. Brooks, M.L. & Brooks, D.L. (2015). Basic Medical Language (5th ed.). Mosby. ISBN: 9780323290487 2. Ogden, S.J., Fluharty, L.K. (2015). Calculation of Drug Dosages (10th ed.). St. Louis, MO: Mosby. ISBN: 978032331069-7(Not included in Mosby/Elsevier package – Purchase separately – Purchase early – Will need this book before semester begins) 3. Weber, J. & Kelley, J. (2014). Health Assessment in Nursing (5th ed.). Lippincott Williams & Wilkins. ISBN: 9781451142808 RECOMMENDED Zerwekh, J, & Gaglione, T. (2011). Mosby’s Assessment memory notecards (2nd ed.). Maryland Heights, MO: Elsevier Mosby. ISBN: 9780323067454 NURS 3530 – Nursing Fundamentals 1. Wilkinson, J.M., Treas, L.S., Barnett, K (2015). Fundamentals of Nursing (Two Volume Set), (3rd ed.). F. A. Davis Company. ISBN: 9780803640771 2. F. A. Davis, (2013). Davis Edge Fundamentals – Access Card (14th ed.). F. A. Davis Company. ISBN: 9780803640238 3. Pagana, K.D. (2013). Mosby’s Manual of Diagnostic and Laboratory Tests (5th ed.). St. Louis, MO: Mosby. ISBN: 9780323089494 4. Myers, E. (2014). RNotes: Nurse’s Clinical Pocket Guide (4th Ed.). -

The Wound/Burn Guidelines –
doi: 10.1111/1346-8138.13288 Journal of Dermatology 2016; : 1–22 GUIDELINE The wound/burn guidelines – 6: Guidelines for the management of burns Yuichiro YOSHINO,1 Mikio OHTSUKA,2 Masakazu KAWAGUCHI,3 Keisuke SAKAI,4 Akira HASHIMOTO,5 Masahiro HAYASHI,3 Naoki MADOKORO,6 Yoshihide ASANO,7 Masatoshi ABE,8 Takayuki ISHII,9 Taiki ISEI,10 Takaaki ITO,11 Yuji INOUE,12 Shinichi IMAFUKU,13 Ryokichi IRISAWA,14 Masaki OHTSUKA,15 Fumihide OGAWA,16 Takafumi KADONO,7 Tamihiro KAWAKAMI,17 Ryuichi KUKINO,18 Takeshi KONO,19 Masanari KODERA,20 Masakazu TAKAHARA,21 Miki TANIOKA,22 Takeshi NAKANISHI,23 Yasuhiro NAKAMURA,24 Minoru HASEGAWA,9 Manabu FUJIMOTO,9 Hiroshi FUJIWARA,25 Takeo MAEKAWA,26 Koma MATSUO,27 Osamu YAMASAKI,15 Andres LE PAVOUX,28 Takao TACHIBANA,29 Hironobu IHN,12 The Wound/Burn Guidelines Committee 1Department of Dermatology, Japanese Red Cross Kumamoto Hospital, Kumamoto, 2Department of Dermatology, Fukushima Medical University, Fukushima, 3Department of Dermatology, Yamagata University Faculty of Medicine, Yamagata, 4Intensive Care Unit, Kumamoto University Hospital, Kumamoto, 5Department of Dermatology, Tohoku University Graduate School of Medicine, Miyagi, 6Department of Dermatology, Mazda Hospital, Hiroshima, 7Department of Dermatology, Faculty of Medicine,University of Tokyo, Tokyo, 8Department of Dermatology, Gunma University Graduate School of Medicine, Gunma, 9Department of Dermatology, Faculty of Medicine, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, Ishikawa, 10Department of Dermatology, Kansai -

Reading List for Diagnostic Radiography Courses, 2021 Entry
Reading List for Diagnostic Radiography courses, 2021 entry BSc (Hons) Diagnostic Radiography BSc (Hons) Diagnostic Radiography with Foundation Ultrasonography BSc (Hons) Diagnostic Radiography with Integrated Foundation Year We are often are asked by students: “…which textbooks should I buy?” There are many different textbooks that are suitable for your studies and, as you progress, other texts will also become important. You are not expected to purchase every title below, and we would encourage you to try them before you buy! Copies of all these books and other electronic resources will be available to you via the School’s “Life Science Resource Centre” (LSRC) and both University and NHS medical libraries. Many students do choose to purchase Clark’s Positioning in Radiography, which is highlighted in yellow in the list below, however, it is not compulsory that you purchase this. Additionally, special purchase offers may occasionally be available from publisher stands at the Freshers’ events when you arrive. If you wish to complete any reading prior to the start date of your course, we would encourage you to look at the HCPC website to look at standards, the HSE website for radiation protection and NICE to see which guidance involves imaging: o https://www.hcpc-uk.org/ o http://www.hse.gov.uk/radiation/ionising/protection.htm o https://www.nice.org.uk/guidance?action=bytreatment&treatments=dia gnostic%20imaging General: Adler, A.M. & Carlton, R.R. (2015) Introduction to Radiologic Sciences and Patient Care, (6th ed.) London: Saunders Elsevier. Ball,J., Moore,A.D., & Turner, S. (2008) Ball and Moore's Essential Physics for Radiographers. -

Table 1B 2005 Community Prescription Numbers, Together with Government
TABLE 1B 2005 COMMUNITY PRESCRIPTION NUMBERS, TOGETHER WITH GOVERNMENT AND PATIENT COSTS FOR PBS-LISTED DRUGS Table 1B includes an estimate of community (non-public hospital) prescription numbers for the 2005 calendar year, costs (government and patient contribution) for the items with a four digit PBS/RPBS code, together with the defined daily dose (DDD) where assigned. There is no cost information available for items with a five digit Amfac drug code. Table 1 exclude the presentation of information on any item with an estimated community use of less than 110 prescriptions in 2005. The prescription items are arranged by ATC code on generic name, and by form and strength using either the PBS drug code (4 digit) or, for non-PBS items, the Amfac drug code (5 digit). Consult the index (page 255) by generic drug name to obtain the appropriate ATC code. An index by 2nd level of the ATC classification follows: ALIMENTARY TRACT AND METABOLISM PAGE NO A01 STOMATOLOGICAL PREPARATIONS 181 A02 DRUGS FOR ACID RELATED DISORDERS 182 A03 DRUGS FOR FUNCTIONAL GASTROINTESTINAL DISORDERS 185 A04 ANTIEMETICS AND ANTINAUSEANTS 187 A05 BILE AND LIVER THERAPY 188 A06 LAXATIVES 189 A07 ANTIDIARRHOEALS, INTESTINAL 191 ANTIINFLAMMATORY/ANTIINFECTIVE AGENTS A08 ANTIOBESITY PREPARATIONS, EXCLUDING DIET PRODUCTS 193 A09 DIGESTIVES, INCLUDING ENZYMES 194 A10 DRUGS USED IN DIABETES 195 A11 VITAMINS 197 A12 MINERAL SUPPLEMENTS 199 A14 ANABOLIC AGENTS FOR SYSTEMIC USE 200 177 BLOOD AND BLOOD FORMING ORGANS B01 ANTITHROMBOTIC AGENTS 201 B02 ANTIHAEMORRHAGICS 203 B03 -

Pharmacy Program and Drug Formulary
Pharmacy Program and Drug Formulary Secure Horizons Group Retiree Medicare Advantage Plan n Pharmacy Program Description n Platinum Plus Enhanced Formulary California Benefits Effective January 1, 2006 Table of Contents Your Secure Horizons Group Retiree Medicare Advantage Plan Prescription Drug Benefit........................................................................................................ iii Secure Horizons Pharmacy Program Definitions .................................................................... iii What Is the Platinum Plus Enhanced Formulary? ....................................................................iv Where to Have Your Prescriptions Filled .................................................................................iv Preferred and Non-Preferred Network Pharmacies .................................................................iv Network Preferred Pharmacy Locations ..................................................................................iv How to Fill a Prescription at a Network Pharmacy ...................................................................v Mail Service Pharmacy ..............................................................................................................v Secure Horizons Group Retiree Medicare Advantage Plan Offers a Two-Part Prescription Drug Benefit ..........................................................................vii Part 1 – Medicare Part D Prescription Drug Coverage ...................................................vii How Your Medicare