CCA Senior Care Options Formulary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Scabies in Healthcare Facilities
Scabies in Healthcare Facilities Tammra L. Morrison, RN BSN Healthcare Associated Infections Coordinator Communicable Disease Branch, Epidemiology Section December 9, 2016 Symptoms • In a person who has never had scabies: • May take 4-6 weeks for symptom onset • In a person who has had scabies in the past: • Symptoms may start in 1-4 days • May be spread PRIOR to symptom onset What to Look for • Intense itching • Especially at night • Pimple-like itchy rash • May affect entire body OR Dermatologie.md common sites: • Wrist, elbow, armpit, webbing between the fingers, nipple, penis, waist, belt-line, and buttocks • Burrows (tunnels) may be seen on the skin • Tiny raised and crooked grayish-white or skin- colored lines Transmission • Direct, prolonged, skin-to-skin contact with an infested person • Sexual partners • Household members • Quick handshake/hug will usually not spread scabies How Long Do Mites Live? • 1-2 months on a person • 48-72 hours off a person • Scabies mites will die at 122 degrees for 10 minutes Webmd.com 5 Diagnosis • Customary appearance and distribution of the rash and presence of burrows. • Confirm diagnosis: • Obtain a skin scraping to examine under a microscope for mites, eggs, or mite fecal matter • Person can still be infested even if mites, eggs, or fecal matter cannot be found • Typically fewer than 10-15 mites present on the entire body • **Crusted scabies may be thousands of mites and should be considered highly contagious** How Do You Treat Scabies? 7 Treatment • Available only by prescription • No "over-the-counter“ -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Bonviva, INN- Ibandronic Acid
European Medicines Agency London, 1 September 2005 Doc.Ref.: EMEA/278873/2005 Bonviva International Nonproprietary Name: Ibandronic acid Following the procedure EMEA/H/C/501/X/01 7 Westferry Circus, Canary Wharf, London E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax (44-20) 74 18 86 68 E-mail: [email protected] http://www.emea.eu.int EMEA 2005 Reproduction and/or distribution of this document is authorised for non commercial purposes only provided the EMEA is acknowledged 1 SCIENTIFIC DISCUSSION 1.1 Introduction and rationale The MAH submitted an extension application under Annex II, point 2 iii to Commission Regulation (EC) No 1085/2003 to request the approval of a 150 mg tablet as a monthly dosing regimen of ibandronate only for the indication of “treatment of osteoporosis in postmenopausal women, in order to reduce the risk of vertebral fractures”. This claim is based on the results of a phase III study MOBILE (BM16549) comparing 100 and 150 mg once monthly to 2.5 mg once daily. Due to the inconveniences associated with intake of oral bisphosphonates (i.e. fasting conditions, frequent upper gastrointestinal intolerance) that may result in poor compliance, it was considered desirable to develop a more convenient drug formulation. Hence a 150 mg once monthly oral regimen is expected to offer greater convenience to postmenopausal women when compared to the currently approved 2.5mg once daily tablet. The development programme No new pre-clinical pharmacodynamic and pharmacokinetic studies have been performed in addition to those included in the previous submission for ibandronate 2.5 mg daily oral tablets. -

Salicylic Acid
Treatment Guide to Common Skin Conditions Prepared by Loren Regier, BSP, BA, Sharon Downey -www.RxFiles.ca Revised: Jan 2004 Dermatitis, Atopic Dry Skin Psoriasis Step 1 - General Treatment Measures Step 1 - General Treatment Measures Step 1 • Avoid contact with irritants or trigger factors • Use cool air humidifiers • Non-pharmacologic measures (general health issues) • Avoid wool or nylon clothing. • Lower house temperature (minimize perspiration) • Moisturizers (will not clear skin, but will ↓ itching) • Wash clothing in soap vs detergent; double rinse/vinegar • Limit use of soap to axillae, feet, and groin • Avoid frequent or prolonged bathing; twice weekly • Topical Steroids Step 2 recommended but daily bathing permitted with • Coal Tar • Colloidal oatmeal bath products adequate skin hydration therapy (apply moisturizer • Anthralin • Lanolin-free water miscible bath oil immediately afterwards) • Vitamin D3 • Intensive skin hydration therapy • Limit use of soap to axillae, feet, and groin • Topical Retinoid Therapy • “Soapless” cleansers for sensitive skin • Apply lubricating emollients such as petrolatum to • Sunshine Step 3 damp skin (e.g. after bathing) • Oral antihistamines (1st generation)for sedation & relief of • Salicylic acid itching give at bedtime +/- a daytime regimen as required Step 2 • Bath additives (tar solns, oils, oatmeal, Epsom salts) • Topical hydrocortisone (0.5%) for inflammation • Colloidal oatmeal bath products Step 2 apply od-tid; ointments more effective than creams • Water miscible bath oil • Phototherapy (UVB) may use cream during day & ointment at night • Humectants: urea, lactic acid, phospholipid • Photochemotherapy (Psoralen + UVA) Step 4 Step 3 • Combination Therapies (from Step 1 & 2 treatments) • Prescription topical corticosteroids: use lowest potency • Oral antihistamines for sedation & relief of itching steroid that is effective and wean to twice weekly. -

Dermatological Effects of Different Keratolytic Agents on Acne Vulgaris
Clin l of ica a l T n r r i u a l o s J Alodeani, J Clin Trials 2016, 6:2 Journal of Clinical Trials DOI: 10.4172/2167-0870.1000262 ISSN: 2167-0870 Research Article Open Access Dermatological Effects of Different Keratolytic Agents on Acne Vulgaris Essa Ajmi Alodeani* College of Medicine at AD-Dwadmi, Shaqra University, Saudi Arabia *Corresponding author: Essa Ajmi Alodeani, College of Medicine at AD-Dwadmi, Shaqra University, Saudi Arabia, Tel: +966 55 075 9042; E-mail: [email protected] Received date: March 24, 2016; Accepted date: April 18, 2016; Published date: April 25, 2016 Copyright: © 2016 Alodeani EA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Acne vulgaris is a chronic inflammatory skin disease; it's one of the most common skin disorders and affects mainly adolescents and young adults. Keratolytic agents are widely used in treatment of acne from several years. In this study we aimed to evaluate and compare the cutaneous response of different keratolytic agents in management of acne vulgaris. Ninety patients were selected among those attending the outpatient dermatology clinic in AD- Dwadmi hospital during the period from October 2015 to February 2016. The selected patients had different forms of acne vulgaris, papulo-pustular, comedonal and post acne scar. Three types of keratolytic agents were used, glycolic acid 50%, salicylic acid 20% and jessner solution. In papulopustular lesions, the three agents were effective with non-significant difference between them; however, there was more excellent results with jessner solution (70 % of patients) then glycolic acid (50%) and lastly salicylic acid (40%). -

Bisphosphonate Use in Patients with Breast Cancer
Bisphosphonate use in patients with breast cancer Information for patients, relatives and carers For more information, please contact: Medicines Information Email: [email protected] York Hospital Medicines Information Tel: 01904 725960 Page 2 Contents Page Why have I been given this leaflet? ............................... 4 How is the treatment given? .......................................... 4 How long will I need to take the treatment for? .............. 6 Can I take other medicines at the same time? ............... 6 Who will prescribe the medication? ............................... 7 What if I am already on bisphosphonate treatment? ...... 7 Bisphosphonates are not licensed for reducing the risk of breast cancer recurrence. What does this mean? ...... 8 Are there any side effects? ............................................ 9 What is osteonecrosis? ............................................... 10 Are there any signs and symptoms I should look out for during treatment? ........................................................ 11 How can I decrease the risk of developing osteonecrosis of the jaw? ................................................................... 12 What do I need to do before starting treatment? .......... 13 Will I require any monitoring, or blood tests? ............... 14 Should I stop taking the bisphosphonate? ................... 14 Tell us what you think of this leaflet ............................. 15 Teaching, training and research ................................... 15 Patient Advice and Liaison Service (PALS) .................. 15 Page 3 Why have I been given this leaflet? You have been given this leaflet because you are going to be treated with a bisphosphonate medicine (zoledronic acid and/or ibandronic acid). In post-menopausal women, with early breast cancer, these drugs have been shown to reduce the risk of the disease recurring and increase length of life. How is the treatment given? The treatment will usually be provided as an oral tablet of ibandronic acid (one 50mg tablet taken once daily). -
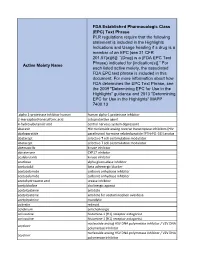
Active Moiety Name FDA Established Pharmacologic Class (EPC) Text
FDA Established Pharmacologic Class (EPC) Text Phrase PLR regulations require that the following statement is included in the Highlights Indications and Usage heading if a drug is a member of an EPC [see 21 CFR 201.57(a)(6)]: “(Drug) is a (FDA EPC Text Phrase) indicated for [indication(s)].” For Active Moiety Name each listed active moiety, the associated FDA EPC text phrase is included in this document. For more information about how FDA determines the EPC Text Phrase, see the 2009 "Determining EPC for Use in the Highlights" guidance and 2013 "Determining EPC for Use in the Highlights" MAPP 7400.13. .alpha. -

Illness Code A
Long Term Illness (LTI) Scheme: Reference Guide as to what is covered in the Health Service Executive for each of the 16 scheduled LTI illnesses. (January 2021) Illness Code C: Cerebral Palsy Antibiotics 1. Doxycycline J01AA02 2. Lymecycline J01AA04 3. Minocycline J01AA08 4. Ampicillin J01CA01 5. Amoxicillin J01CA04 6. Azlocillin J01CA09 7. Benzylpenicillin J01CE01 8. Phenoxymethylpenicillin J01CE02 9. Flucloxacillin J01CF05 10. Amoxicillin & Enzyme Inhibitor J01CR02 11. Cefalexin J01DB01 12. Cefuroxime J01DC02 13. Cefaclor J01DC04 14. Ceftazidime J01DD02 15. Ceftriaxone J01DD04 16. Cefixime J01DD08 17. Cefpodoxime J01DD13 18. Trimethoprim J01EA01 19. Sulfamethoxazole & Trimethoprim J01EE01 20. Erythromycin J01FA01 21. Clarithromycin J01FA09 22. Azithromycin J01FA10 23. Clindamycin J01FF01 24. Gentamicin J01GB03 25. Amikacin J01GB06 26. Ofloxacin J01MA01 27. Ciprofloxacin J01MA02 28. Levofloxacin J01MA12 29. Moxifloxacin J01MA14 30. Vancomycin J01XA01 31. Teicoplanin J01XA02 32. Colistin J01XB01 33. Fusidic Acid J01XC01 34. Nitrofurantoin J01XE01 Muscle relaxants 1. Baclofen M03BX01 2. Tizanidine M03BX02 3. Dantrolene M03CA01 Anticonvulsants (including Benzodiazepines used for this purpose) 1. Phenobarbital N03AA02 2. Primidone N03AA03 page 1 3. Phenytoin N03AB02 4. Ethosuximide N03AD01 5. Clonazepam N03AE01 6. Carbamazepine N03AF01 7. Oxcarbazepine N03AF02 8. Rufinamide N03AF03 9. Eslicarbazepine N03AF04 10. Valproic Acid N03AG01 11. Vigabatrin N03AG04 12. Tiagabine N03AG06 13. Lamotrigine N03AX09 14. Topiramate N03AX11 15. Gabapentin N03AX12 16. Levetiracetam N03AX14 17. Zonisamide N03AX15 18. Pregabalin N03AX16 19. Lacosamide N03AX18 20. Retigabine N03AX21 21. Perampanel N03AX22 22. Diazepam (Rectal) N05BA01 23. Clobazam N05BA09 24. Midazolam N05CD08 25. Acetazolamide S01EC01 Anxiolytics 1. Diazepam N05BA01 2. Chlordiazepoxide N05BA02 3. Lorazepam N05BA06 4. Bromazepam N05BA08 5. Clobazam N05BA09 6. Prazepam N05BA11 7. Alprazolam N05BA12 Hypnotics 8. -

Oral Alitretinoin in Congenital Ichthyosis: a Pilot Study Shows Variable Effects and a Risk of Central Hypothyroidism
Included in the theme issue: 256 Letters to the Editor ACNE, RETINOIDS AND LYMPHOMAS Acta Derm Venereol 2012; 92: 256–257 Oral Alitretinoin in Congenital Ichthyosis: A Pilot Study Shows Variable Effects and a Risk of Central Hypothyroidism Agneta Gånemo1, Mette Sommerlund2 and Anders Vahlquist3* 1Department of Dermatology, Institute of Clinical Research in Malmö, Lund University, Skåne University Hospital, Malmö, Sweden, 2Department of Dermatology and Venereology, Aarhus University Hospital, Aarhus, Denmark, and 3Department of Medical Sciences (Dermatology), Uppsala University, Uppsala, Sweden. E-mail: [email protected] Accepted October 20, 2011. Congenital ichthyosis is a large group of hereditary skin planned treatment period of 3 months. Clinical and laboratory disorders with different aetiologies, all of which are pre- evaluations were performed before the start of therapy and at monthly intervals, and consisted of physical examination, pho- sent at birth (1). The patients have dry, widespread scaling tography, interviewing the patients about effects and side-effects and thickened skin (2). At present there is no cure for of therapy, and blood sampling for analysis of haematological ichthyosis and therapy is mostly symptomatic. Life-long parameters, liver enzymes, creatinine, cholesterol, triglycerides, treatment with emollients is essential, and some patients thyroxin (T4) and tyroid-stimulating hormone (TSH) levels. also use systemic therapy with retinoids, especially aci- tretin (3). The most serious adverse effect of retinoids is RESULTS teratogenicity, which is a special concern for acitretin as it is excreted from the body slowly (3). All 4 patients completed the 3-month long trial, and two Alitretinoin (9-cis retinoic acid) is a fairly new oral of them (nos 1 and 3) wished to continue alitretinoin retinoid with more rapid clearance than acitretin. -

History of Formulary Changes Contents
History of Formulary Changes Pre-Single PDL Changes (before October 1, 2020) Revised for 11/1/2020 Contents Medicaid Health Plan Common Formulary Changes Effective October 1, 2020 ................................................................................................................................... 2 Medicaid Health Plan Common Formulary Changes Effective July 1, 2020 .......................................................................................................................................... 4 Medicaid Health Plan Common Formulary Changes Effective April 1, 2020 ........................................................................................................................................ 6 Medicaid Health Plan Common Formulary Changes Effective January 1, 2020 .................................................................................................................................... 8 Medicaid Health Plan Common Formulary Changes Effective October 1, 2019 ................................................................................................................................. 10 Medicaid Health Plan Common Formulary Changes Effective July 1, 2019 ........................................................................................................................................ 12 Medicaid Health Plan Common Formulary Changes Effective April 1, 2019 ...................................................................................................................................... 14 -
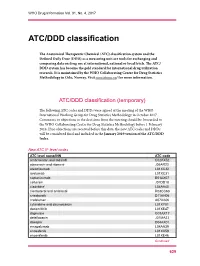
ATC/DDD Classification
WHO Drug Information Vol. 31, No. 4, 2017 ATC/DDD classification The Anatomical Therapeutic Chemical (ATC) classification system and the Defined Daily Dose (DDD) as a measuring unit are tools for exchanging and comparing data on drug use at international, national or local levels. The ATC/ DDD system has become the gold standard for international drug utilization research. It is maintained by the WHO Collaborating Centre for Drug Statistics Methodology in Oslo, Norway. Visit www.whocc.no/ for more information. ATC/DDD classification (temporary) The following ATC codes and DDDs were agreed at the meeting of the WHO International Working Group for Drug Statistics Methodology in October 2017. Comments or objections to the decisions from the meeting should be forwarded to the WHO Collaborating Centre for Drug Statistics Methodology before 1 February 2018. If no objections are received before this date, the new ATC codes and DDDs will be considered final and included in the January 2019 version of the ATC/DDD Index. New ATC 5th level codes ATC level name/INN ATC code ambrisentan and tadalafil C02KX52 atazanavir and ritonavir J05AR23 atezolizumab L01XC32 avelumab L01XC31 caplacizumab B01AX07 cefteram J01DD18 cladribine1 L04AA40 clenbuterol and ambroxol R03CC63 crisaborole D11AH06 crofelemer A07XA06 cytarabine and daunorubicin L01XY01 dacomitinib L01XE47 dapivirine G01AX17 delafloxacin J01MA23 doxepin D04AX01 emapalumab L04AA39 enasidenib L01XX59 encorafenib L01XE46 Continued 629 ATC/DDD classification WHO Drug Information Vol. 31, No. 4, 2017 -

ED227273.Pdf
DOCUMENT RESUft ED 227 273 y CE 035 300 ' TITLE APharmacy Spicialist, Militkry Curriculum Materials for Vocation47 andileChlaical Education. INSTITuTION Air Force Training Command, Sheppa* AFB, Tex.; Ohio State Univ., Columbus. Natfonal Center for Research in Vocational Education. SPONS AGENCY Office of Education (DHEW)x Washington, D.C. PUB DATE 18 Jul 75 NOTE 774p.; Some pages are marginally legible. ,PUB TYPE Guides - Classroom Use Guides (For Teachers) (052), , EDRS ?RICE 14P05/PC31 Plus Postage. ` DESCRIPTORS Behavioral Objectives; Course Descriptions; A Curriculum Guides; Drug Abuse; Drug,Therapy; 4Drug Use; Learning Activities; Lesson Plans; *Pharmaceutical Education; Pharmacists; *Pharmacology; *Pharmacy; Postsecondary Education; Programed Instructional Materials; Textbooks; Workbooks IDENTIFIERS. Military CuFr.iculum Project liBSTRACT These teacher and studdnt,materials for a . postsecondary-level course in pharmacy comprise one of a numberof military-developed curriculum packages selected for adaptation to voCational instruction 'and curriculum dei7elopment in acivilian setting. The purpose stated for the 256-hour course iS totrain students in the basic technical phases of pharmacy and theminimum essential knowledge and skills necessaryior.the compounding and - dispensing of drugs, the economical operation of a pharmacy,and the proper use of drugs, chemicals, andbiological products. The course consists of three blocks of instruction. Block I contains four, lessons: pharmaceutical calculations I and laboratory,inorganic chemistry, and organic chemistry. The five lessons in Block II cover anatomy ,and physiology, introduction topharmacoloe, toxicology, drug abuse, and pharmaceutical and medicinal agents. Block III provides five lessons: phdrmaceutical calculations\I and II, techniques"of pharmaceutical compounding, pharmaceutiCal dosage for s, and compounding laboratbry. Instructormaterials include a cb se chart, lesson plans, and aplan of instruction detailing instructional,bnits, criterion objectives, lesson duration,and support materials needed.