ATC/DDD Classification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Separation of Β-Receptor Blockers and Analogs by Capillary Liquid Chromatography
ORIGINAL ARTICLES College of Pharmaceutical Sciences, Zhejiang University, Hangzhou, China Separation of b-receptor blockers and analogs by Capillary Liquid Chromatography (CLC) and Pressurized Capillary Electrochromatography (pCEC) using a vancomycin chiral stationary phase column Zhongyi Chen, Su Zeng, Tongwei Yao Received September 15, 2006, accepted October 28, 2006 Tongwei Jao, Department of Pharmaceutical Analysis and Drug Metabolism, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou, 310031, China [email protected] Pharmazie 62: 585–592 (2007) doi: 10.1691/ph.2007.8.6194 Enantiomeric separation of chiral pharmaceuticals was carried out by means of in capillary liquid chroma- tography (CLC) and pressurized capillary electrochromatography (pCEC) using a vancomycin chiral sta- tionary phase (CSP). A 100 mm I.D. fused-silica capillary was packed with 5 mm diameter silica particles modified with vancomycin. Enantiomeric resolution of fifteen b-receptor blockers and analogs was stu- died by polar organic CLC mode and reversed-phase pCEC mode using mobile phases containing methanol-isopropanol-acetic acid-triethylamine and TEAA buffer-methanol, respectively. Several factors affecting chiral separation were investigated in both CLC and pCEC mode. Good enantiomeric resolution was achieved by CLC mode for propranolol, celiprolol, esmolol, bisoprolol, atenolol, metoprolol and car- teolol using methanol-isopropanol-acetic acid-triethylamine (70 : 30 : 0.05 : 0.05, v/v/v/v) as mobile phase and for clenbuterol, bambuterol, terbutaline, and salbutamol using methanol-isopropanol-acetic acid- triethylamine (50 : 50 : 0.05 : 005 or 50 : 50: 0.025 : 0.05, v/v/v/v) as mobile phase. The baseline was achieved by pCEC mode for the separation of esmolol, bisoprolol, atenolol, metoprolol, carteolol in the mobile phase containing MeOH-0.05%TEAA (pH 7.0) (90 : 10, v/v) (–10 kV), and that of propranolol and celiprolol in the mobile phase containing MeOH-0.025%TEAA (pH 7.0) (90 : 10, v/v)(–10 kV). -

Clenbuterol Human Effects the Effect of Clenbuterol in Humans Is Researched Through Examining the History and Regulations of the Drug
Clenbuterol Human Effects The effect of clenbuterol in humans is researched through examining the history and regulations of the drug. Specifically, Alberto Contador’s case is considered. Tag Words: Clenbuterol; drugs; Beta-2 Agonist; Effects; Thermogenic; Fat; Harmful; Authors: Jessie Yeh, Horace Lau, Danielle Lovisone with Julie M. Fagan, Ph.D. Summary (written by Danielle Lovisone) As a sympathomimetic and Beta-2 agonist, clenbuterol have several deleterious effects on the human body. The drug acts as a thermogenic stimulant, increasing lean muscle mass and respiratory efficiency while reducing fat. Cases on clenbuterol, including animal tests and human occurrences, support these unnatural and potentially harmful effects. With this, athletes and body builders have recently increased their use of the drug. Particularly, Alberto Contador has recently been targeted for having traces of clenbuterol in a urine drug test. Contador claims, instead of doping, this trace amount was unknowingly received from ingesting beef in Spain during the 2010 Tour de France. Although clenbuterol is banned in most areas of the world, this explanation seems plausible because the drug is poorly regulated by organizations such as the FDA. To examine Contador’s case further, our group compiled research on clenbuterol to ultimately hypothesize that Contador received this trace amount from contaminated beef. Our findings were submitted to the World Anti-Doping Agency as part of our Service Project. Video Link Class project 2010 fall: www.youtube.com/watch?v=ZTeJiBvbLhA The Issue: Clenbuterol The Effects of Clenbuterol on the Human Body By Jessie Yeh What is Clenbuterol? Clenbuterol is a chemical compound closely resembling the structure of an amine. -

Bonviva, INN- Ibandronic Acid
European Medicines Agency London, 1 September 2005 Doc.Ref.: EMEA/278873/2005 Bonviva International Nonproprietary Name: Ibandronic acid Following the procedure EMEA/H/C/501/X/01 7 Westferry Circus, Canary Wharf, London E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax (44-20) 74 18 86 68 E-mail: [email protected] http://www.emea.eu.int EMEA 2005 Reproduction and/or distribution of this document is authorised for non commercial purposes only provided the EMEA is acknowledged 1 SCIENTIFIC DISCUSSION 1.1 Introduction and rationale The MAH submitted an extension application under Annex II, point 2 iii to Commission Regulation (EC) No 1085/2003 to request the approval of a 150 mg tablet as a monthly dosing regimen of ibandronate only for the indication of “treatment of osteoporosis in postmenopausal women, in order to reduce the risk of vertebral fractures”. This claim is based on the results of a phase III study MOBILE (BM16549) comparing 100 and 150 mg once monthly to 2.5 mg once daily. Due to the inconveniences associated with intake of oral bisphosphonates (i.e. fasting conditions, frequent upper gastrointestinal intolerance) that may result in poor compliance, it was considered desirable to develop a more convenient drug formulation. Hence a 150 mg once monthly oral regimen is expected to offer greater convenience to postmenopausal women when compared to the currently approved 2.5mg once daily tablet. The development programme No new pre-clinical pharmacodynamic and pharmacokinetic studies have been performed in addition to those included in the previous submission for ibandronate 2.5 mg daily oral tablets. -

CCA Senior Care Options Formulary
Commonwealth Care Alliance Senior Care Option HMO SNP 2021 List of Covered Drugs Formulary 30 Winter Street • Boston, MA 02108 PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN This formulary was updated on 08/01/2021. For more recent information or other questions, please contact Senior Care Options Program (HMO SNP) Member Services, at 1-866-610-2273 or, for TTY users, 711, 8 a.m. – 8 p.m., 7 days a week, or visit www.commonwealthcaresco.org. HPMS Approved Formulary File Submission ID 00021589, Version Number 13 Senior Care Options Program (HMO SNP) 2021 Formulary (List of Covered Drugs) PLEASE READ: THIS DO CUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN HPMS Approved Formulary File Submission ID 00021589, Version Number 13 Note to existing members: This formulary has changed since last year. Please review this document to make sure that it still contains the drugs you take. When this drug list (formulary) refers to “we,” “us”, or “our,” it means Commonwealth Care Alliance. When it refers to “plan” or “our plan,” it means 2021 Senior Care Options Program. This document includes list of the drugs (formulary) for our plan which is current as of 08/01/2021. This formulary document applies to all SCO members. For an updated formulary, please contact us. Our contact information, along with the date we last updated the formulary, appears on the front and back cover pages. You must generally use network pharmacies to use your prescription drug benefit. Benefits, formulary, pharmacy n etwork, and/or copayments/coinsurance may change on January 1, 2022, and from time to time during the year. -

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances
WHO/PSM/QSM/2006.3 The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances 2006 Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Medicines Policy and Standards The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -

Bisphosphonate Use in Patients with Breast Cancer
Bisphosphonate use in patients with breast cancer Information for patients, relatives and carers For more information, please contact: Medicines Information Email: [email protected] York Hospital Medicines Information Tel: 01904 725960 Page 2 Contents Page Why have I been given this leaflet? ............................... 4 How is the treatment given? .......................................... 4 How long will I need to take the treatment for? .............. 6 Can I take other medicines at the same time? ............... 6 Who will prescribe the medication? ............................... 7 What if I am already on bisphosphonate treatment? ...... 7 Bisphosphonates are not licensed for reducing the risk of breast cancer recurrence. What does this mean? ...... 8 Are there any side effects? ............................................ 9 What is osteonecrosis? ............................................... 10 Are there any signs and symptoms I should look out for during treatment? ........................................................ 11 How can I decrease the risk of developing osteonecrosis of the jaw? ................................................................... 12 What do I need to do before starting treatment? .......... 13 Will I require any monitoring, or blood tests? ............... 14 Should I stop taking the bisphosphonate? ................... 14 Tell us what you think of this leaflet ............................. 15 Teaching, training and research ................................... 15 Patient Advice and Liaison Service (PALS) .................. 15 Page 3 Why have I been given this leaflet? You have been given this leaflet because you are going to be treated with a bisphosphonate medicine (zoledronic acid and/or ibandronic acid). In post-menopausal women, with early breast cancer, these drugs have been shown to reduce the risk of the disease recurring and increase length of life. How is the treatment given? The treatment will usually be provided as an oral tablet of ibandronic acid (one 50mg tablet taken once daily). -
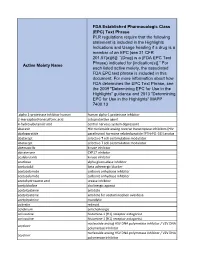
Active Moiety Name FDA Established Pharmacologic Class (EPC) Text
FDA Established Pharmacologic Class (EPC) Text Phrase PLR regulations require that the following statement is included in the Highlights Indications and Usage heading if a drug is a member of an EPC [see 21 CFR 201.57(a)(6)]: “(Drug) is a (FDA EPC Text Phrase) indicated for [indication(s)].” For Active Moiety Name each listed active moiety, the associated FDA EPC text phrase is included in this document. For more information about how FDA determines the EPC Text Phrase, see the 2009 "Determining EPC for Use in the Highlights" guidance and 2013 "Determining EPC for Use in the Highlights" MAPP 7400.13. .alpha. -

Skeletal and Cardiac Muscle Ergogenics and Side Effects Of
edicine s M & rt D o o p p i S n f g o S Douillard, J Sport Medic Doping Studie 2011, S1 l Journal of Sports Medicine & Doping t a u n d r i e u DOI: 10.4172/2161-0673.S1-001 s o J Studies ISSN: 2161-0673 Review Article Open Access Skeletal and Cardiac Muscle Ergogenics and Side Effects of Clenbuterol Treatment Aymeric Douillard* INRA, UMR866 Dynamique Musculaire et Métabolisme, Université Montpellier 1, F-34060 Montpellier, France Abstract Well known, well detected and still used by athletes, clenbuterol is one of the β2-agonists which has no authorization for therapeutic use, contrarily to salbutamol, salmeterol and formoterol in the 2012 World Anti-Doping Agency list. However, clenbuterol is still detected in athletes’ antidoping test samples. Its ability to induce muscle hypertrophy but also its strong lipolytic action and the absence of androgenic effects have made it a prized substances by athletes, specially females, without scruples whose performance requires significant muscle strength. Like the effects of clenbuterol on the heart, the effects of clenbuterol on skeletal muscle are dependent on the doses used and duration of the treatment. If there is a consensus concerning the clenbuterol action on the phenotypic conversion from slow to fast type fibers and on the hypertrophy, there is, to our knowledge, no consensus concerning the effects of clenbuterol on the slow type fibers and slow profile muscles. There is also no consensus concerning the clenbuterol effects on performance. We will shortly reviewing the known operating mode, side and benefits effects of short and long term β-agonists, and specially clenbuterol, treatment on mammals. -

Illness Code A
Long Term Illness (LTI) Scheme: Reference Guide as to what is covered in the Health Service Executive for each of the 16 scheduled LTI illnesses. (January 2021) Illness Code C: Cerebral Palsy Antibiotics 1. Doxycycline J01AA02 2. Lymecycline J01AA04 3. Minocycline J01AA08 4. Ampicillin J01CA01 5. Amoxicillin J01CA04 6. Azlocillin J01CA09 7. Benzylpenicillin J01CE01 8. Phenoxymethylpenicillin J01CE02 9. Flucloxacillin J01CF05 10. Amoxicillin & Enzyme Inhibitor J01CR02 11. Cefalexin J01DB01 12. Cefuroxime J01DC02 13. Cefaclor J01DC04 14. Ceftazidime J01DD02 15. Ceftriaxone J01DD04 16. Cefixime J01DD08 17. Cefpodoxime J01DD13 18. Trimethoprim J01EA01 19. Sulfamethoxazole & Trimethoprim J01EE01 20. Erythromycin J01FA01 21. Clarithromycin J01FA09 22. Azithromycin J01FA10 23. Clindamycin J01FF01 24. Gentamicin J01GB03 25. Amikacin J01GB06 26. Ofloxacin J01MA01 27. Ciprofloxacin J01MA02 28. Levofloxacin J01MA12 29. Moxifloxacin J01MA14 30. Vancomycin J01XA01 31. Teicoplanin J01XA02 32. Colistin J01XB01 33. Fusidic Acid J01XC01 34. Nitrofurantoin J01XE01 Muscle relaxants 1. Baclofen M03BX01 2. Tizanidine M03BX02 3. Dantrolene M03CA01 Anticonvulsants (including Benzodiazepines used for this purpose) 1. Phenobarbital N03AA02 2. Primidone N03AA03 page 1 3. Phenytoin N03AB02 4. Ethosuximide N03AD01 5. Clonazepam N03AE01 6. Carbamazepine N03AF01 7. Oxcarbazepine N03AF02 8. Rufinamide N03AF03 9. Eslicarbazepine N03AF04 10. Valproic Acid N03AG01 11. Vigabatrin N03AG04 12. Tiagabine N03AG06 13. Lamotrigine N03AX09 14. Topiramate N03AX11 15. Gabapentin N03AX12 16. Levetiracetam N03AX14 17. Zonisamide N03AX15 18. Pregabalin N03AX16 19. Lacosamide N03AX18 20. Retigabine N03AX21 21. Perampanel N03AX22 22. Diazepam (Rectal) N05BA01 23. Clobazam N05BA09 24. Midazolam N05CD08 25. Acetazolamide S01EC01 Anxiolytics 1. Diazepam N05BA01 2. Chlordiazepoxide N05BA02 3. Lorazepam N05BA06 4. Bromazepam N05BA08 5. Clobazam N05BA09 6. Prazepam N05BA11 7. Alprazolam N05BA12 Hypnotics 8. -

WO 2014/141069 Al 18 September 2014 (18.09.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/141069 Al 18 September 2014 (18.09.2014) P O P C T (51) International Patent Classification: Novartis Pharmaceuticals Corporation, 150 Industrial A61K 9/00 (2006.01) A61K 31/00 (2006.01) Road, San Carlos, California 94070 (US). A61K 9/16 (2006.01) (74) Agent: MAZZA, Michael; Novartis Vaccines and Dia (21) International Application Number: gnostics, Inc., 4560 Horton Street, Emeryville, California PCT/IB2014/059632 94608 (US). (22) International Filing Date: (81) Designated States (unless otherwise indicated, for every 11 March 2014 ( 11.03.2014) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (25) Filing Language: English BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (26) Publication Language: English DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (30) Priority Data: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 61/784,865 14 March 2013 (14.03.2013) US MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (71) Applicant (for all designated States except US) : NO¬ OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, VARTIS AG [CH/CH]; Lichtstrasse 35, CH-4056 Basel SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (CH). -

2-Adrenergic Receptor Is Determined by Conformational Equilibrium in the Transmembrane Region
ARTICLE Received 21 Mar 2012 | Accepted 2 Aug 2012 | Published 4 Sep 2012 DOI: 10.1038/ncomms2046 Efficacy of theβ 2-adrenergic receptor is determined by conformational equilibrium in the transmembrane region Yutaka Kofuku1,2, Takumi Ueda1, Junya Okude1, Yutaro Shiraishi1, Keita Kondo1, Masahiro Maeda3, Hideki Tsujishita3 & Ichio Shimada1,4 Many drugs that target G-protein-coupled receptors (GPCRs) induce or inhibit their signal transduction with different strengths, which affect their therapeutic properties. However, the mechanism underlying the differences in the signalling levels is still not clear, although several structures of GPCRs complexed with ligands determined by X-ray crystallography are available. Here we utilized NMR to monitor the signals from the methionine residue at position 82 in neutral antagonist- and partial agonist-bound states of β2-adrenergic receptor (β2AR), which are correlated with the conformational changes of the transmembrane regions upon activation. We show that this residue exists in a conformational equilibrium between the inverse agonist- bound states and the full agonist-bound state, and the population of the latter reflects the signal transduction level in each ligand-bound state. These findings provide insights into the multi-level signalling of β2AR and other GPCRs, including the basal activity, and the mechanism of signal transduction mediated by GPCRs. 1 Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo 7-3-1, Bunkyo-ku, Tokyo 113-0033, Japan. 2 Japan Biological Informatics Consortium (JBIC), Tokyo 135-0064, Japan. 3 Shionogi Co., Ltd., Discovery Research Laboratories, Osaka 561-0825, Japan. 4 Biomedicinal Information Research Center (BIRC), National Institute of Advanced Industrial Science and Technology (AIST), Aomi 2-41-6, Koto-ku, Tokyo 135-0064, Japan. -

PRAC Draft Agenda of Meeting 11-14 May 2020
11 May 2020 EMA/PRAC/257460/2020 Human Division Pharmacovigilance Risk Assessment Committee (PRAC) Draft agenda for the meeting on 11-14 May 2020 Chair: Sabine Straus – Vice-Chair: Martin Huber 11 May 2020, 10:30 – 19:30, via teleconference 12 May 2020, 08:30 – 19:30, via teleconference 13 May 2020, 08:30 – 19:30, via teleconference 14 May 2020, 08:30 – 16:00, via teleconference Organisational, regulatory and methodological matters (ORGAM) 28 May 2020, 09:00-12:00, via teleconference Disclaimers Some of the information contained in this agenda is considered commercially confidential or sensitive and therefore not disclosed. With regard to intended therapeutic indications or procedure scopes listed against products, it must be noted that these may not reflect the full wording proposed by applicants and may also change during the course of the review. Additional details on some of these procedures will be published in the PRAC meeting highlights once the procedures are finalised. Of note, this agenda is a working document primarily designed for PRAC members and the work the Committee undertakes. Note on access to documents Some documents mentioned in the agenda cannot be released at present following a request for access to documents within the framework of Regulation (EC) No 1049/2001 as they are subject to on-going procedures for which a final decision has not yet been adopted. They will become public when adopted or considered public according to the principles stated in the Agency policy on access to documents (EMA/127362/2006, Rev. 1). Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2020.