Table 1 2009 Community Prescription Numbers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidance on Identification of Alternatives to New Pops
Stockholm Convention on Persistent Organic Pollutants Guidance on identification of alternatives to new POPs Secretariat of the Stockholm Convention Concept of “Substitution” under the Stockholm Convention • The substitution is a strategy promoted by the Stockholm Convention to reach its objectives • Parties that are still producing or using the new POPs listed in Annex A, will need to search and identify alternatives to replace them • In the case of PFOS and for the exemptions for uses allowed by the Convention, these group of chemicals will be eventually prohibited and Parties are therefore encouraged to find alternatives to substitute them 2 Availability of alternatives • Currently, some countries have phased out the use of some of the new POPs, and there are feasible alternatives available to replace them Alternatives Chemical Name Use Ethoprop, oxamyl Pesticide to control banana root borer Cyfluthrin, Imidacloprid Pesticide to control tobacco wireworms Azadirachtin, bifenthrin, boric acid, carbaryl, Pesticide to control capsaicin, cypermethrin, cyfluthrin, ants and/or deltamethrin, diazinon, dichlorvos, cockroaches esfenvalerate, imidacloprid, lamda-cyhalothrin, Chlordecone malathion, permethrin, piperonyl butoxide, pyrethrins, pyriproxyfen, resmethrin, s- bioallerthrin, tetramethrin Bacillus thuringiensis, cultural practices such Pest management as crop rotation, intercropping, and trap cropping; barrier methods, such as screens, and bagging of fruit; use of traps such as pheromone and light traps to attract and kill insects. 3 -

WHO Drug Information Vol. 12, No. 3, 1998
WHO DRUG INFORMATION VOLUME 12 NUMBER 3 • 1998 RECOMMENDED INN LIST 40 INTERNATIONAL NONPROPRIETARY NAMES FOR PHARMACEUTICAL SUBSTANCES WORLD HEALTH ORGANIZATION • GENEVA Volume 12, Number 3, 1998 World Health Organization, Geneva WHO Drug Information Contents Seratrodast and hepatic dysfunction 146 Meloxicam safety similar to other NSAIDs 147 Proxibarbal withdrawn from the market 147 General Policy Issues Cholestin an unapproved drug 147 Vigabatrin and visual defects 147 Starting materials for pharmaceutical products: safety concerns 129 Glycerol contaminated with diethylene glycol 129 ATC/DDD Classification (final) 148 Pharmaceutical excipients: certificates of analysis and vendor qualification 130 ATC/DDD Classification Quality assurance and supply of starting (temporary) 150 materials 132 Implementation of vendor certification 134 Control and safe trade in starting materials Essential Drugs for pharmaceuticals: recommendations 134 WHO Model Formulary: Immunosuppressives, antineoplastics and drugs used in palliative care Reports on Individual Drugs Immunosuppresive drugs 153 Tamoxifen in the prevention and treatment Azathioprine 153 of breast cancer 136 Ciclosporin 154 Selective serotonin re-uptake inhibitors and Cytotoxic drugs 154 withdrawal reactions 136 Asparaginase 157 Triclabendazole and fascioliasis 138 Bleomycin 157 Calcium folinate 157 Chlormethine 158 Current Topics Cisplatin 158 Reverse transcriptase activity in vaccines 140 Cyclophosphamide 158 Consumer protection and herbal remedies 141 Cytarabine 159 Indiscriminate antibiotic -

Lindane – Product Discontinuation • the FDA Announced the Discontinuation of Lindane 1% Lotion and 1% Shampoo Due to Product Line Rationalization
Lindane – Product Discontinuation • The FDA announced the discontinuation of Lindane 1% lotion and 1% shampoo due to product line rationalization. — The discontinuation is not due to product quality, safety or efficacy concerns. • Lindane lotion is indicated for the treatment of scabies (infestations of Sarcoptes scabei) only in patients who cannot tolerate other approved therapies, or have failed treatment with other approved therapies. • Lindane shampoo is indicated for the treatment of head lice (infestations of Pediculus humanus capitis), crab lice (infestations of Pthirus pubis), and their ova only in patients who cannot tolerate other approved therapies, or have failed treatment with other approved therapies. • Other prescription products indicated to treat lice include Ulesfia® (benzyl alcohol) 5% lotion, Ovide® (malathion) 0.5% lotion, Natroba™ (spinosad) 0.9% topical suspension, and Sklice® (ivermectin) 0.5% lotion. — Over-the-counter products indicated to treat lice include various formulations of 1% permethrin (eg, Nix®), various formulations of piperonyl butoxide/pyrethrins (eg, CareOne® Lice), and other miscellaneous products such as Lycelle®. • Other prescription products indicated to treat scabies include Eurax® (crotamiton) 10% cream and 10% lotion and Elimite™ (permethrin) 5% cream. optumrx.com OptumRx® specializes in the delivery, clinical management and affordability of prescription medications and consumer health products. We are an Optum® company — a leading provider of integrated health services. Learn more at optum.com. All Optum® trademarks and logos are owned by Optum, Inc. All other brand or product names are trademarks or registered marks of their respective owners. This document contains information that is considered proprietary to OptumRx and should not be reproduced without the express written consent of OptumRx. -

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

The Impact of a Changed Legislation on Reporting of Adverse Drug Reactions in Sweden, with Focus on Nurses Reporting
The impact of a changed legislation on reporting of adverse drug reactions in Sweden, with focus on nurses reporting Sofia A. Karlsson, Ingela Jacobsson, Marit Danell Boman, Katja M. Hakkarainen, Henrik Lövborg, Staffan Hägg and Anna K Jönsson Linköping University Post Print N.B.: When citing this work, cite the original article. The original publication is available at www.springerlink.com: Sofia A. Karlsson, Ingela Jacobsson, Marit Danell Boman, Katja M. Hakkarainen, Henrik Lövborg, Staffan Hägg and Anna K Jönsson, The impact of a changed legislation on reporting of adverse drug reactions in Sweden, with focus on nurses reporting, 2015, European Journal of Clinical Pharmacology, (71), 5, 631-636. http://dx.doi.org/10.1007/s00228-015-1839-6 Copyright: Springer Verlag (Germany) http://www.springerlink.com/?MUD=MP Postprint available at: Linköping University Electronic Press http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-118037 The impact of a changed legislation on reporting of adverse drug reactions in Sweden, with focus on nurses’ reporting Sofia A Karlsson1, Ingela Jacobsson2, Marit Danell Boman3, Katja M Hakkarainen4,5, Henrik Lövborg2, Staffan Hägg2,5, Anna K Jönsson2 Affiliations: 1. Department of Public Health and Community Medicine, the Sahlgrenska Academy at University of Gothenburg, Gothenburg, Sweden 2. Department of Clinical Pharmacology and Department of Medical and Health Sciences, Linköping University, Linköping, Sweden 3. Division of Clinical Pharmacology, University Hospital of Umeå, Umeå, Sweden 4. Nordic School of -

Crohn's & Colitis Program, Patient Information Guide
Inflammatory Bowel Disease Program Patient Information Guide Page # Contents ..........................................................................................................................................1 Welcome! Welcome Letter ....................................................................................................................3 Important Things to Know Up Front ....................................................................................4 Meet Your Crohn’s & Colitis Team .....................................................................................6 How to Contact Us Crohn’s & Colitis Clinic Schedule .....................................................................................11 Physician and Nurse Contact Information..........................................................................12 Appointment Scheduling and Other Contact Information..................................................13 The Basics of Inflammatory Bowel Disease (IBD) Basic Information about Inflammatory Bowel Disease (IBD) ...........................................14 Frequently Asked Questions about Inflammatory Bowel Disease (IBD) ..........................18 Testing in IBD Colonoscopy and Flexible Sigmoidoscopy ........................................................................20 Upper Endoscopy ...............................................................................................................21 Capsule Endoscopy and Deep Enteroscopy .......................................................................22 -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group R01 (Nasal preparations) Table of Contents Page INTRODUCTION 5 DISCLAIMER 7 GLOSSARY OF TERMS USED IN THIS DOCUMENT 8 ACTIVE SUBSTANCES Cyclopentamine (ATC: R01AA02) 10 Ephedrine (ATC: R01AA03) 11 Phenylephrine (ATC: R01AA04) 14 Oxymetazoline (ATC: R01AA05) 16 Tetryzoline (ATC: R01AA06) 19 Xylometazoline (ATC: R01AA07) 20 Naphazoline (ATC: R01AA08) 23 Tramazoline (ATC: R01AA09) 26 Metizoline (ATC: R01AA10) 29 Tuaminoheptane (ATC: R01AA11) 30 Fenoxazoline (ATC: R01AA12) 31 Tymazoline (ATC: R01AA13) 32 Epinephrine (ATC: R01AA14) 33 Indanazoline (ATC: R01AA15) 34 Phenylephrine (ATC: R01AB01) 35 Naphazoline (ATC: R01AB02) 37 Tetryzoline (ATC: R01AB03) 39 Ephedrine (ATC: R01AB05) 40 Xylometazoline (ATC: R01AB06) 41 Oxymetazoline (ATC: R01AB07) 45 Tuaminoheptane (ATC: R01AB08) 46 Cromoglicic Acid (ATC: R01AC01) 49 2 Levocabastine (ATC: R01AC02) 51 Azelastine (ATC: R01AC03) 53 Antazoline (ATC: R01AC04) 56 Spaglumic Acid (ATC: R01AC05) 57 Thonzylamine (ATC: R01AC06) 58 Nedocromil (ATC: R01AC07) 59 Olopatadine (ATC: R01AC08) 60 Cromoglicic Acid, Combinations (ATC: R01AC51) 61 Beclometasone (ATC: R01AD01) 62 Prednisolone (ATC: R01AD02) 66 Dexamethasone (ATC: R01AD03) 67 Flunisolide (ATC: R01AD04) 68 Budesonide (ATC: R01AD05) 69 Betamethasone (ATC: R01AD06) 72 Tixocortol (ATC: R01AD07) 73 Fluticasone (ATC: R01AD08) 74 Mometasone (ATC: R01AD09) 78 Triamcinolone (ATC: R01AD11) 82 -

Hormonal Treatment Strategies Tailored to Non-Binary Transgender Individuals
Journal of Clinical Medicine Review Hormonal Treatment Strategies Tailored to Non-Binary Transgender Individuals Carlotta Cocchetti 1, Jiska Ristori 1, Alessia Romani 1, Mario Maggi 2 and Alessandra Daphne Fisher 1,* 1 Andrology, Women’s Endocrinology and Gender Incongruence Unit, Florence University Hospital, 50139 Florence, Italy; [email protected] (C.C); jiska.ristori@unifi.it (J.R.); [email protected] (A.R.) 2 Department of Experimental, Clinical and Biomedical Sciences, Careggi University Hospital, 50139 Florence, Italy; [email protected]fi.it * Correspondence: fi[email protected] Received: 16 April 2020; Accepted: 18 May 2020; Published: 26 May 2020 Abstract: Introduction: To date no standardized hormonal treatment protocols for non-binary transgender individuals have been described in the literature and there is a lack of data regarding their efficacy and safety. Objectives: To suggest possible treatment strategies for non-binary transgender individuals with non-standardized requests and to emphasize the importance of a personalized clinical approach. Methods: A narrative review of pertinent literature on gender-affirming hormonal treatment in transgender persons was performed using PubMed. Results: New hormonal treatment regimens outside those reported in current guidelines should be considered for non-binary transgender individuals, in order to improve psychological well-being and quality of life. In the present review we suggested the use of hormonal and non-hormonal compounds, which—based on their mechanism of action—could be used in these cases depending on clients’ requests. Conclusion: Requests for an individualized hormonal treatment in non-binary transgender individuals represent a future challenge for professionals managing transgender health care. For each case, clinicians should balance the benefits and risks of a personalized non-standardized treatment, actively involving the person in decisions regarding hormonal treatment. -

Inflammatory Bowel Disease Agents
Therapeutic Class Overview Inflammatory Bowel Disease Agents INTRODUCTION • Inflammatory bowel disease (IBD) is a spectrum of chronic idiopathic inflammatory intestinal conditions that cause gastrointestinal symptoms including diarrhea, abdominal pain, bleeding, fatigue, and weight loss. The exact cause of IBD is unknown; however, proposed etiologies involve a combination of infectious, genetic, and lifestyle factors (Bernstein et al 2015, Peppercorn 2019[a], Peppercorn 2020[c]). • Complications of IBD include hemorrhage, rectal fissures, fistulas, peri-rectal and intra-abdominal abscesses, and colon cancer. Possible extra-intestinal complications include hepatobiliary complications, anemia, arthritis and arthralgias, uveitis, skin lesions, and mood and anxiety disorders (Bernstein et al 2015). • Ulcerative colitis (UC) and Crohn’s disease (CD) are 2 forms of IBD that differ in pathophysiology and presentation; as a result of these differences, the approach to the treatment of each condition often differs (Peppercorn 2019[a]). • UC is characterized by recurrent episodes of inflammation of the mucosal layer of the colon. The inflammation, limited to the mucosa, commonly involves the rectum and may extend in a proximal and continuous fashion to affect other parts of the colon. The hallmark clinical symptom is an inflamed rectum with symptoms of urgency, bleeding, and tenesmus (Peppercorn 2020[c], Rubin et al 2019). • CD can involve any part of the gastrointestinal tract and is characterized by transmural inflammation and “skip areas.” Transmural inflammation may lead to fibrosis, strictures, sinus tracts, and fistulae (Peppercorn 2019[b]). • The immune system is known to play a critical role in the underlying pathogenesis of IBD. It is suggested that abnormal responses of both innate and adaptive immunity mechanisms induce aberrant intestinal tract inflammation in IBD patients (Geremia et al 2014). -

Assessing the Availability, Service Quality, and Price of Essential Medicines In
Assessing the Availability, Service Quality, and Price of Essential Medicines in Private Pharmacies in Afghanistan Norio Kasahara A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2015 Reading Committee: Louis P. Garrison, Jr., Chair Joseph B. Babigumira Andy Stergachis Program Authorized to Offer Degree: Pharmaceutical Outcomes Research and Policy ©Copyright 2015 Norio Kasahara ii Table of Contents Abstract ................................................................................................................................................................................... ................................................................................................ ................................................................................................ .................................................................................. ............... vvv Acknowledgements ................................................................................................................................................................................... ................................................................................................ ................................................................................. ............ viiviivii Summary ................................................................................................................................................................................... ............................................................................................... -

COMPARISON of the WHO ATC CLASSIFICATION & Ephmra/Intellus Worldwide ANATOMICAL CLASSIFICATION
COMPARISON OF THE WHO ATC CLASSIFICATION & EphMRA/Intellus Worldwide ANATOMICAL CLASSIFICATION: VERSION June 2019 2 Comparison of the WHO ATC Classification and EphMRA / Intellus Worldwide Anatomical Classification The following booklet is designed to improve the understanding of the two classification systems. The development of the two systems had previously taken place separately. EphMRA and WHO are now working together to ensure that there is a convergence of the 2 systems rather than a divergence. In order to better understand the two classification systems, we should pay attention to the way in which substances/products are classified. WHO mainly classifies substances according to the therapeutic or pharmaceutical aspects and in one class only (particular formulations or strengths can be given separate codes, e.g. clonidine in C02A as antihypertensive agent, N02C as anti-migraine product and S01E as ophthalmic product). EphMRA classifies products, mainly according to their indications and use. Therefore, it is possible to find the same compound in several classes, depending on the product, e.g., NAPROXEN tablets can be classified in M1A (antirheumatic), N2B (analgesic) and G2C if indicated for gynaecological conditions only. The purposes of classification are also different: The main purpose of the WHO classification is for international drug utilisation research and for adverse drug reaction monitoring. This classification is recommended by the WHO for use in international drug utilisation research. The EphMRA/Intellus Worldwide classification has a primary objective to satisfy the marketing needs of the pharmaceutical companies. Therefore, a direct comparison is sometimes difficult due to the different nature and purpose of the two systems. -
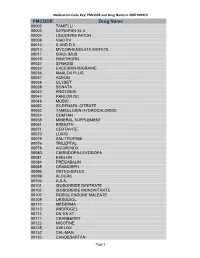
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS Cdc-Pdf
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00002 TAMIFLU 00003 DITROPAN XL II 00004 LIDODERM PATCH 00008 VIACTIV 00010 A AND D II 00013 MYCOPHENOLATE MOFETIL 00017 SIROLIMUS 00019 HAWTHORN 00027 SYNAGIS 00032 EXCEDRIN MIGRAINE 00036 MAALOX PLUS 00037 ACEON 00038 GLYSET 00039 SONATA 00042 PROTONIX 00044 PANLOR DC 00048 MOBIC 00052 SILDENAFIL CITRATE 00053 TAMSULOSIN HYDROCHLORIDE 00054 COMTAN 00058 MINERAL SUPPLEMENT 00061 BISMUTH 00071 CERTAVITE 00073 LUXIQ 00075 SAL-TROPINE 00076 TRILEPTAL 00078 AGGRENOX 00080 CARBIDOPA-LEVODOPA 00081 EXELON 00084 PREGABALIN 00085 ORAMORPH 00096 OSTEO-BIFLEX 00099 ALOCRIL 00100 A.S.A. 00101 ISOSORBIDE DINITRATE 00102 ISOSORBIDE MONONITRATE 00107 ROSIGLITAZONE MALEATE 00109 URSODIOL 00112 MEDERMA 00113 ANDROGEL 00114 DILTIA XT 00117 CRANBERRY 00123 NICOTINE 00125 AVELOX 00132 CAL-MAG 00133 CANDESARTAN Page 1 Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00148 PROLIXIN D 00149 D51/2 NS 00150 NICODERM CQ PATCH 00151 TUSSIN 00152 CEREZYME 00154 CHILDREN'S IBUPROFEN 00156 PROPOXACET-N 00159 KALETRA 00161 BISOPROLOL 00167 NOVOLIN N 00169 KETOROLAC TROMETHAMINE 00172 OPHTHALMIC OINTMENT 00173 ELA-MAX 00176 PREDNISOLONE ACETATE 00179 COLLOID SILVER 00184 KEPPRA 00187 OPHTHALMIC DROPS 00190 ABDEC 00191 HAPONAL 00192 SPECTRAVITE 00198 ENOXAPARIN SODIUM 00206 ACTONEL 00208 CELECOXIB 00209 GLUCOVANCE 00211 LEVALL 5.0 00213 PANTOPRAZOLE SODIUM 00217 TEMODAR 00218 CARBAMIDE PEROXIDE 00221 CHINESE HERBAL MEDS 00224 MILK AND MOLASSES ENEMA 00238 ZOLMITRIPTAN 00239