Open Formulary for Web W Spclty V2 (Updated 11-11-2016).Xlsx
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Al Exposure Increases Proline Levels by Different Pathways in An
www.nature.com/scientificreports OPEN Al exposure increases proline levels by diferent pathways in an Al‑sensitive and an Al‑tolerant rye genotype Alexandra de Sousa1,2, Hamada AbdElgawad2,4, Fernanda Fidalgo1, Jorge Teixeira1, Manuela Matos3, Badreldin A. Hamed4, Samy Selim5, Wael N. Hozzein6, Gerrit T. S. Beemster2 & Han Asard2* Aluminium (Al) toxicity limits crop productivity, particularly at low soil pH. Proline (Pro) plays a role in protecting plants against various abiotic stresses. Using the relatively Al‑tolerant cereal rye (Secale cereale L.), we evaluated Pro metabolism in roots and shoots of two genotypes difering in Al tolerance, var. RioDeva (sensitive) and var. Beira (tolerant). Most enzyme activities and metabolites of Pro biosynthesis were analysed. Al induced increases in Pro levels in each genotype, but the mechanisms were diferent and were also diferent between roots and shoots. The Al‑tolerant genotype accumulated highest Pro levels and this stronger increase was ascribed to simultaneous activation of the ornithine (Orn)‑biosynthetic pathway and decrease in Pro oxidation. The Orn pathway was particularly enhanced in roots. Nitrate reductase (NR) activity, N levels, and N/C ratios demonstrate that N‑metabolism is less inhibited in the Al‑tolerant line. The correlation between Pro changes and diferences in Al‑sensitivity between these two genotypes, supports a role for Pro in Al tolerance. Our results suggest that diferential responses in Pro biosynthesis may be linked to N‑availability. Understanding the role of Pro in diferences between genotypes in stress responses, could be valuable in plant selection and breeding for Al resistance. Proline (Pro) is involved in a wide range of plant physiological and developmental processes1. -

Formaldehyde? Formaldehyde Is a Colorless, Strong-Smelling Gas Used to Make Household Products and Building Materials, Furniture, and Paper Products
What is formaldehyde? Formaldehyde is a colorless, strong-smelling gas used to make household products and building materials, furniture, and paper products. It is used in particleboard, plywood, and fiberboard. What products contain formaldehyde? Formaldehyde can be found in most homes and buildings. Formaldehyde is also released into the air from many products you may use in your home. Because formaldehyde breaks down in air, you may breathe it in from such products as • carpet cleaner • gas cookers and open fireplaces, • cosmetics, • glue, • fabric softeners, • household cleaners, and • fingernail polish and hardeners, • latex paint. Burning cigarettes and other tobacco products also release formaldehyde. Products give off different amounts of formaldehyde. For example, • fingernail polish gives off more formaldehyde than do plywood and new carpet, and • some paper products—such as grocery bags and paper towels—give off only small amounts of formaldehyde. Our bodies even produce some formaldehyde, although only in small amounts. Will I get sick if I breathe or touch formaldehyde? You might not get sick if you breathe or touch formaldehyde, but if you have breathed or touched formaldehyde you may have symptoms such as • sore, itchy, or burning eyes, nose, or throat; • skin rash; or • breathing symptoms such as chest tightness, coughing, and shortness of breath. People who are more likely to get sick from being around formaldehyde are children, the elderly, and people with asthma. Formaldehyde may affect children more than it does adults. If you think your child may have been around formaldehyde, and he or she has symptoms contact a doctor. You should also know that: babies are not likely to get formaldehyde from breast milk, and you may be more sensitive to formaldehyde if you have asthma. -

Method 323—Measurement of Formaldehyde Emissions from Natural Gas-Fired Stationary Sources—Acetyl Acetone Derivitization Method
While we have taken steps to ensure the accuracy of this Internet version of the document, it is not the official version. Please refer to the official version in the FR publication, which appears on the Government Printing Office's FDSys website (http://www.gpo.gov/fdsys/browse/collectionCfr.action?). Method 323—Measurement of Formaldehyde Emissions From Natural Gas-Fired Stationary Sources—Acetyl Acetone Derivitization Method 1.0 Introduction. This method describes the sampling and analysis procedures of the acetyl acetone colorimetric method for measuring formaldehyde emissions in the exhaust of natural gas-fired, stationary combustion sources. This method, which was prepared by the Gas Research Institute (GRI), is based on the Chilled Impinger Train Method for Methanol, Acetone, Acetaldehyde, Methyl Ethyl Ketone, and Formaldehyde (Technical Bulletin No. 684) developed and published by the National Council of the Paper Industry for Air and Stream Improvement, Inc. (NCASI). However, this method has been prepared specifically for formaldehyde and does not include specifications (e.g., equipment and supplies) and procedures (e.g., sampling and analytical) for methanol, acetone, acetaldehyde, and methyl ethyl ketone. To obtain reliable results, persons using this method should have a thorough knowledge of at least Methods 1 and 2 of 40 CFR part 60, appendix A–1; Method 3 of 40 CFR part 60, appendix A–2; and Method 4 of 40 CFR part 60, appendix A–3. 1.1 Scope and Application 1.1.1 Analytes. The only analyte measured by this method is formaldehyde (CAS Number 50–00–0). 1.1.2 Applicability. This method is for analyzing formaldehyde emissions from uncontrolled and controlled natural gas-fired, stationary combustion sources. -

Toxicological Profile for Formaldehyde
TOXICOLOGICAL PROFILE FOR FORMALDEHYDE U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry July 1999 FORMALDEHYDE ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. FORMALDEHYDE iii UPDATE STATEMENT Toxicological profiles are revised and republished as necessary, but no less than once every three years. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology/Toxicology Information Branch 1600 Clifton Road NE, E-29 Atlanta, Georgia 30333 FORMALDEHYDE vii QUICK REFERENCE FOR HEALTH CARE PROVIDERS Toxicological Profiles are a unique compilation of toxicological information on a given hazardous substance. Each profile reflects a comprehensive and extensive evaluation, summary, and interpretation of available toxicologic and epidemiologic information on a substance. Health care providers treating patients potentially exposed to hazardous substances will find the following information helpful for fast answers to often-asked questions. Primary Chapters/Sections of Interest Chapter 1: Public Health Statement: The Public Health Statement can be a useful tool for educating patients about possible exposure to a hazardous substance. It explains a substance’s relevant toxicologic properties in a nontechnical, question-and-answer format, and it includes a review of the general health effects observed following exposure. Chapter 2: Health Effects: Specific health effects of a given hazardous compound are reported by route of exposure, by type of health effect (death, systemic, immunologic, reproductive), and by length of exposure (acute, intermediate, and chronic). -

1.0 Introduction. This Method Describes the Sampling and Analysis Procedures of the Acetyl Acetone Colorimetric Method For
Method 323 8/7/2017 While we have taken steps to ensure the accuracy of this Internet version of the document, it is not the official version. To see a complete version including any recent edits, visit: https://www.ecfr.gov/cgi-bin/ECFR?page=browse and search under Title 40, Protection of Environment. METHOD 323—MEASUREMENT OF FORMALDEHYDE EMISSIONS FROM NATURAL GAS-FIRED STATIONARY SOURCES—ACETYL ACETONE DERIVITIZATION METHOD 1.0 Introduction. This method describes the sampling and analysis procedures of the acetyl acetone colorimetric method for measuring formaldehyde emissions in the exhaust of natural gas-fired, stationary combustion sources. This method, which was prepared by the Gas Research Institute (GRI), is based on the Chilled Impinger Train Method for Methanol, Acetone, Acetaldehyde, Methyl Ethyl Ketone, and Formaldehyde (Technical Bulletin No. 684) developed and published by the National Council of the Paper Industry for Air and Stream Improvement, Inc. (NCASI). However, this method has been prepared specifically for formaldehyde and does not include specifications (e.g., equipment and supplies) and procedures (e.g., sampling and analytical) for methanol, acetone, acetaldehyde, and methyl ethyl ketone. To obtain reliable results, persons using this method should have a thorough knowledge of at least Methods 1 and 2 of 40 CFR part 60, appendix A-1; Method 3 of 40 CFR part 60, appendix A-2; and Method 4 of 40 CFR part 60, appendix A-3. 1.1 Scope and Application 1.1.1 Analytes. The only analyte measured by this method is formaldehyde (CAS Number 50- 00-0). 1.1.2 Applicability. -

WHO Guidelines for Indoor Air Quality : Selected Pollutants
WHO GUIDELINES FOR INDOOR AIR QUALITY WHO GUIDELINES FOR INDOOR AIR QUALITY: WHO GUIDELINES FOR INDOOR AIR QUALITY: This book presents WHO guidelines for the protection of pub- lic health from risks due to a number of chemicals commonly present in indoor air. The substances considered in this review, i.e. benzene, carbon monoxide, formaldehyde, naphthalene, nitrogen dioxide, polycyclic aromatic hydrocarbons (especially benzo[a]pyrene), radon, trichloroethylene and tetrachloroethyl- ene, have indoor sources, are known in respect of their hazard- ousness to health and are often found indoors in concentrations of health concern. The guidelines are targeted at public health professionals involved in preventing health risks of environmen- SELECTED CHEMICALS SELECTED tal exposures, as well as specialists and authorities involved in the design and use of buildings, indoor materials and products. POLLUTANTS They provide a scientific basis for legally enforceable standards. World Health Organization Regional Offi ce for Europe Scherfi gsvej 8, DK-2100 Copenhagen Ø, Denmark Tel.: +45 39 17 17 17. Fax: +45 39 17 18 18 E-mail: [email protected] Web site: www.euro.who.int WHO guidelines for indoor air quality: selected pollutants The WHO European Centre for Environment and Health, Bonn Office, WHO Regional Office for Europe coordinated the development of these WHO guidelines. Keywords AIR POLLUTION, INDOOR - prevention and control AIR POLLUTANTS - adverse effects ORGANIC CHEMICALS ENVIRONMENTAL EXPOSURE - adverse effects GUIDELINES ISBN 978 92 890 0213 4 Address requests for publications of the WHO Regional Office for Europe to: Publications WHO Regional Office for Europe Scherfigsvej 8 DK-2100 Copenhagen Ø, Denmark Alternatively, complete an online request form for documentation, health information, or for per- mission to quote or translate, on the Regional Office web site (http://www.euro.who.int/pubrequest). -
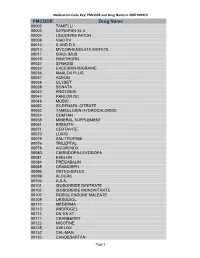
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS Cdc-Pdf
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00002 TAMIFLU 00003 DITROPAN XL II 00004 LIDODERM PATCH 00008 VIACTIV 00010 A AND D II 00013 MYCOPHENOLATE MOFETIL 00017 SIROLIMUS 00019 HAWTHORN 00027 SYNAGIS 00032 EXCEDRIN MIGRAINE 00036 MAALOX PLUS 00037 ACEON 00038 GLYSET 00039 SONATA 00042 PROTONIX 00044 PANLOR DC 00048 MOBIC 00052 SILDENAFIL CITRATE 00053 TAMSULOSIN HYDROCHLORIDE 00054 COMTAN 00058 MINERAL SUPPLEMENT 00061 BISMUTH 00071 CERTAVITE 00073 LUXIQ 00075 SAL-TROPINE 00076 TRILEPTAL 00078 AGGRENOX 00080 CARBIDOPA-LEVODOPA 00081 EXELON 00084 PREGABALIN 00085 ORAMORPH 00096 OSTEO-BIFLEX 00099 ALOCRIL 00100 A.S.A. 00101 ISOSORBIDE DINITRATE 00102 ISOSORBIDE MONONITRATE 00107 ROSIGLITAZONE MALEATE 00109 URSODIOL 00112 MEDERMA 00113 ANDROGEL 00114 DILTIA XT 00117 CRANBERRY 00123 NICOTINE 00125 AVELOX 00132 CAL-MAG 00133 CANDESARTAN Page 1 Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00148 PROLIXIN D 00149 D51/2 NS 00150 NICODERM CQ PATCH 00151 TUSSIN 00152 CEREZYME 00154 CHILDREN'S IBUPROFEN 00156 PROPOXACET-N 00159 KALETRA 00161 BISOPROLOL 00167 NOVOLIN N 00169 KETOROLAC TROMETHAMINE 00172 OPHTHALMIC OINTMENT 00173 ELA-MAX 00176 PREDNISOLONE ACETATE 00179 COLLOID SILVER 00184 KEPPRA 00187 OPHTHALMIC DROPS 00190 ABDEC 00191 HAPONAL 00192 SPECTRAVITE 00198 ENOXAPARIN SODIUM 00206 ACTONEL 00208 CELECOXIB 00209 GLUCOVANCE 00211 LEVALL 5.0 00213 PANTOPRAZOLE SODIUM 00217 TEMODAR 00218 CARBAMIDE PEROXIDE 00221 CHINESE HERBAL MEDS 00224 MILK AND MOLASSES ENEMA 00238 ZOLMITRIPTAN 00239 -

Formaldehyde
Poison Facts: High Chemicals: Formaldehyde Properties of the Chemical Formaldehyde is a colorless gas at ordinary temperatures. It has a pungent, suffocating odor. The chemical is very reactive and combines readily with many substances. It is miscible with water, acetone, benzene, diethyl ether, chloroform and ethanol. Formalin is a solution of about 37 percent formaldehyde by weight in water, usually with 10 to 15 percent methanol added to prevent polymerization. This solution is full-strength and also known as Formalin 100 percent or Formalin 40, which signifies that it contains 40 grams of formaldehyde within 100 ml of the solution. Uses of the Chemical Formaldehyde is used in fertilizers, insecticides, germicides, fungicides, herbicides, sewage treatment, paper-making preservatives, embalming fluids, disinfectants, ureaformaldehyde resins, foam insulation, industrial and soil Stalinist, urea and melamine resins, polyacetal and phenolic resins, artificial silk and cellulose esters, dye fasteners, explosives, latex-backed fabrics, particle board, plywood, air fresheners, cosmetics, wet fingernail hardeners and polishes, antimicrobial hair shampoos and conditioners, water-based paints, chemicals for tanning and preserving hides, and as a chemical intermediate. Absorption, Distribution, Metabolism and Excretion (ADME) Formaldehyde is rapidly absorbed by ingestion and inhalation. Delayed absorption of methanol might occur following ingestion of formalin if the formaldehyde causes fixation of the stomach. Based on studies, very little formaldehyde is absorbed through the dermal route. In all cases, absorption appears to be limited to cell layers immediately adjacent to the point of contact. Formaldehyde is rapidly metabolized to formic acid, largely in the liver by the catalytic action of alcohol dehydrogenase and to a lesser extent in erythrocytes in the brain, kidney and muscles. -

Advanced Textiles for Wound Care
Woodhead Publishing in Textiles: Number 85 Advanced textiles for wound care Edited by S. Rajendran Oxford Cambridge New Delhi © 2009 Woodhead Publishing Limited The Textile Institute and Woodhead Publishing The Textile Institute is a unique organisation in textiles, clothing and footwear. Incorporated in England by a Royal Charter granted in 1925, the Institute has individual and corporate members in over 90 countries. The aim of the Institute is to facilitate learning, recognise achievement, reward excellence and disseminate information within the global textiles, clothing and footwear industries. Historically, The Textile Institute has published books of interest to its members and the textile industry. To maintain this policy, the Institute has entered into partnership with Woodhead Publishing Limited to ensure that Institute members and the textile industry continue to have access to high calibre titles on textile science and technology. Most Woodhead titles on textiles are now published in collaboration with The Textile Institute. Through this arrangement, the Institute provides an Editorial Board which advises Woodhead on appropriate titles for future publication and suggests possible editors and authors for these books. Each book published under this arrangement carries the Institute’s logo. Woodhead books published in collaboration with The Textile Institute are offered to Textile Institute members at a substantial discount. These books, together with those published by The Textile Institute that are still in print, are offered on the Woodhead web site at: www.woodheadpublishing.com. Textile Institute books still in print are also available directly from the Institute’s website at: www.textileinstitutebooks.com. A list of Woodhead books on textile science and technology, most of which have been published in collaboration with The Textile Institute, can be found at the end of the contents pages. -

Sources of Formaldehyde in Bountiful, Utah
atmosphere Article Sources of Formaldehyde in Bountiful, Utah Nitish Bhardwaj 1, Ariel Kelsch 1, Delbert J. Eatough 1, Ryan Thalman 2 , Nancy Daher 3, Kerry Kelly 4 , Isabel Cristina Jaramillo 4 and Jaron C. Hansen 1,* 1 Department of Chemistry and Biochemistry, Brigham Young University, Provo, UT 84602, USA; [email protected] (N.B.); [email protected] (A.K.); [email protected] (D.J.E.) 2 Department of Chemistry, Snow College, Richfield, UT 84701, USA; [email protected] 3 Utah Division of Air Quality, Salt Lake City, UT 84116, USA; [email protected] 4 Chemical Engineering, University of Utah, Salt Lake City, UT 84112, USA; [email protected] (K.K.); [email protected] (I.C.J.) * Correspondence: [email protected]; Tel.: +1-801-422-4066 Abstract: The U.S Environmental Protection Agency’s National Air Toxics Trends Stations Network has been measuring the concentration of hazardous air pollutants (HAPs) including formaldehyde (HCHO) since 2003. Bountiful, Utah (USA) has served as one of the urban monitoring sites since the network was established. Starting in 2013, the mean concentration of HCHO measured in Bountiful, Utah exceeded the non-cancer risk threshold and the 1 in 1 million cancer risk threshold. In addition, the measured concentrations were more than double those found at surrounding locations in Utah. A Positive Matrix Factorization (PMF) analysis using PMF-EPA v5 was performed using historical data (2004–2017) to better understand the sources of formaldehyde in the region. The historical data set included samples that were collected every sixth day on a 24 h basis. -

Amino Acid Catalyzed Direct Asymmetric Aldol Reactions: a Bioorganic Approach to Catalytic Asymmetric Carbon-Carbon Bond-Forming Reactions
5260 J. Am. Chem. Soc. 2001, 123, 5260-5267 Amino Acid Catalyzed Direct Asymmetric Aldol Reactions: A Bioorganic Approach to Catalytic Asymmetric Carbon-Carbon Bond-Forming Reactions Kandasamy Sakthivel, Wolfgang Notz, Tommy Bui, and Carlos F. Barbas III* Contribution from The Skaggs Institute for Chemical Biology and the Department of Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037 ReceiVed January 3, 2001 Abstract: Direct asymmetric catalytic aldol reactions have been successfully performed using aldehydes and unmodified ketones together with commercially available chiral cyclic secondary amines as catalysts. Structure- based catalyst screening identified L-proline and 5,5-dimethyl thiazolidinium-4-carboxylate (DMTC) as the most powerful amino acid catalysts for the reaction of both acyclic and cyclic ketones as aldol donors with aromatic and aliphatic aldehydes to afford the corresponding aldol products with high regio-, diastereo-, and enantioselectivities. Reactions employing hydroxyacetone as an aldol donor provide anti-1,2-diols as the major product with ee values up to >99%. The reactions are assumed to proceed via a metal-free Zimmerman- Traxler-type transition state and involve an enamine intermediate. The observed stereochemistry of the products is in accordance with the proposed transition state. Further supporting evidence is provided by the lack of nonlinear effects. The reactions tolerate a small amount of water (<4 vol %), do not require inert reaction conditions and preformed enolate equivalents, and can be conveniently performed at room temperature in various solvents. In addition, reaction conditions that facilitate catalyst recovery as well as immobilization are described. Finally, mechanistically related addition reactions such as ketone additions to imines (Mannich- type reactions) and to nitro-olefins and R,â-unsaturated diesters (Michael-type reactions) have also been developed. -

Proline- and Alanine-Rich N-Terminal Extension of the Basic Bovine P-Crystallin B1 Chains
CORE Metadata, citation and similar papers at core.ac.uk Provided by Elsevier - Publisher Connector Volume 161, number 2 FEBS 0790 September 1983 Proline- and alanine-rich N-terminal extension of the basic bovine P-crystallin B1 chains G.A.M. Berbers, W.A. Hoekman, H. Bloemendal, W.W. de Jong, T. Kleinschmidt* and G. Braunitzer* Laboratorium voor Biochemie, Universiteit van Nijmegen, Geert Grooteplein Noord 21, 6525 EZ Nijmegen, The Netherlands and *Max-Planck-Institut ft’ir Biochemie, Abteilung Proteinchemie, D-8033 Martinsried bei Miinchen, FRG Received 22 June 1983 The amino acid sequence of the N-terminal region of the two basic bovine &crystallin B1 chains has been analyzed. The results reveal that @Bibis derived in vivo from the primary gene product @la by removal of a short N-terminal sequence. It appears that them1 chains have the same domain structure as observed in other /3- and y-crystallin chains. They have, however, a very long N-terminal extension in comparison with other &chains. This extension is mainly composed of a remarkable Pro- and Ala-rich sequence, which suggests an interaction of these structural proteins with the cytoskeleton and/or the plasma membranes of the lens cells. Protein sequence Bovine &crystallin N-terminal extension Proline- and aianine-rich Domain structure 1. INTRODUCTION 33 000 and 31000, respectively, are characteristic for fltikt, [ 111. ,8Fha is a primary gene product, from The crystallins are evolutionary highly conserv- which ,8&b arises by post-translational modifica- ed structural eye lens proteins, which can be divid; tion [lo-121, most probably a proteolytic step ed into 4 classes: (Y-,,&, y- and t-crystallin [ 11.