Antimicrobial Dressing for Diabetic Foot Ulcer Colonized with MRSA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recent Advances in Developing Insect Natural Products As Potential Modern Day Medicines
Hindawi Publishing Corporation Evidence-Based Complementary and Alternative Medicine Volume 2014, Article ID 904958, 21 pages http://dx.doi.org/10.1155/2014/904958 Review Article Recent Advances in Developing Insect Natural Products as Potential Modern Day Medicines Norman Ratcliffe,1,2 Patricia Azambuja,3 and Cicero Brasileiro Mello1 1 Laboratorio´ de Biologia de Insetos, Departamento de Biologia Geral, Universidade Federal Fluminense, Niteroi,´ RJ, Brazil 2 Department of Biosciences, College of Science, Swansea University, Singleton Park, Swansea SA2 8PP, UK 3 Laboratorio´ de Bioqu´ımica e Fisiologia de Insetos, Instituto Oswaldo Cruz, Fundac¸ao˜ Oswaldo Cruz, Avenida Brasil 4365, 21045-900 Rio de Janeiro, RJ, Brazil Correspondence should be addressed to Patricia Azambuja; [email protected] Received 1 December 2013; Accepted 28 January 2014; Published 6 May 2014 Academic Editor: Ronald Sherman Copyright © 2014 Norman Ratcliffe et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Except for honey as food, and silk for clothing and pollination of plants, people give little thought to the benefits of insects in their lives. This overview briefly describes significant recent advances in developing insect natural products as potential new medicinal drugs. This is an exciting and rapidly expanding new field since insects are hugely variable and have utilised an enormous range of natural products to survive environmental perturbations for 100s of millions of years. There is thus a treasure chest of untapped resources waiting to be discovered. Insects products, such as silk and honey, have already been utilised for thousands of years, and extracts of insects have been produced for use in Folk Medicine around the world, but only with the development of modern molecular and biochemical techniques has it become feasible to manipulate and bioengineer insect natural products into modern medicines. -

Myiasis During Adventure Sports Race
DISPATCHES reexamined 1 day later and was found to be largely healed; Myiasis during the forming scar remained somewhat tender and itchy for 2 months. The maggot was sent to the Finnish Museum of Adventure Natural History, Helsinki, Finland, and identified as a third-stage larva of Cochliomyia hominivorax (Coquerel), Sports Race the New World screwworm fly. In addition to the New World screwworm fly, an important Old World species, Mikko Seppänen,* Anni Virolainen-Julkunen,*† Chrysoimya bezziana, is also found in tropical Africa and Iiro Kakko,‡ Pekka Vilkamaa,§ and Seppo Meri*† Asia. Travelers who have visited tropical areas may exhibit aggressive forms of obligatory myiases, in which the larvae Conclusions (maggots) invasively feed on living tissue. The risk of a Myiasis is the infestation of live humans and vertebrate traveler’s acquiring a screwworm infestation has been con- animals by fly larvae. These feed on a host’s dead or living sidered negligible, but with the increasing popularity of tissue and body fluids or on ingested food. In accidental or adventure sports and wildlife travel, this risk may need to facultative wound myiasis, the larvae feed on decaying tis- be reassessed. sue and do not generally invade the surrounding healthy tissue (1). Sterile facultative Lucilia larvae have even been used for wound debridement as “maggot therapy.” Myiasis Case Report is often perceived as harmless if no secondary infections In November 2001, a 41-year-old Finnish man, who are contracted. However, the obligatory myiases caused by was participating in an international adventure sports race more invasive species, like screwworms, may be fatal (2). -
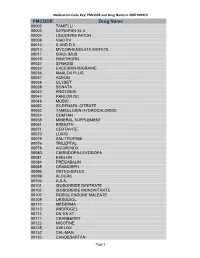
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS Cdc-Pdf
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00002 TAMIFLU 00003 DITROPAN XL II 00004 LIDODERM PATCH 00008 VIACTIV 00010 A AND D II 00013 MYCOPHENOLATE MOFETIL 00017 SIROLIMUS 00019 HAWTHORN 00027 SYNAGIS 00032 EXCEDRIN MIGRAINE 00036 MAALOX PLUS 00037 ACEON 00038 GLYSET 00039 SONATA 00042 PROTONIX 00044 PANLOR DC 00048 MOBIC 00052 SILDENAFIL CITRATE 00053 TAMSULOSIN HYDROCHLORIDE 00054 COMTAN 00058 MINERAL SUPPLEMENT 00061 BISMUTH 00071 CERTAVITE 00073 LUXIQ 00075 SAL-TROPINE 00076 TRILEPTAL 00078 AGGRENOX 00080 CARBIDOPA-LEVODOPA 00081 EXELON 00084 PREGABALIN 00085 ORAMORPH 00096 OSTEO-BIFLEX 00099 ALOCRIL 00100 A.S.A. 00101 ISOSORBIDE DINITRATE 00102 ISOSORBIDE MONONITRATE 00107 ROSIGLITAZONE MALEATE 00109 URSODIOL 00112 MEDERMA 00113 ANDROGEL 00114 DILTIA XT 00117 CRANBERRY 00123 NICOTINE 00125 AVELOX 00132 CAL-MAG 00133 CANDESARTAN Page 1 Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00148 PROLIXIN D 00149 D51/2 NS 00150 NICODERM CQ PATCH 00151 TUSSIN 00152 CEREZYME 00154 CHILDREN'S IBUPROFEN 00156 PROPOXACET-N 00159 KALETRA 00161 BISOPROLOL 00167 NOVOLIN N 00169 KETOROLAC TROMETHAMINE 00172 OPHTHALMIC OINTMENT 00173 ELA-MAX 00176 PREDNISOLONE ACETATE 00179 COLLOID SILVER 00184 KEPPRA 00187 OPHTHALMIC DROPS 00190 ABDEC 00191 HAPONAL 00192 SPECTRAVITE 00198 ENOXAPARIN SODIUM 00206 ACTONEL 00208 CELECOXIB 00209 GLUCOVANCE 00211 LEVALL 5.0 00213 PANTOPRAZOLE SODIUM 00217 TEMODAR 00218 CARBAMIDE PEROXIDE 00221 CHINESE HERBAL MEDS 00224 MILK AND MOLASSES ENEMA 00238 ZOLMITRIPTAN 00239 -

Advanced Textiles for Wound Care
Woodhead Publishing in Textiles: Number 85 Advanced textiles for wound care Edited by S. Rajendran Oxford Cambridge New Delhi © 2009 Woodhead Publishing Limited The Textile Institute and Woodhead Publishing The Textile Institute is a unique organisation in textiles, clothing and footwear. Incorporated in England by a Royal Charter granted in 1925, the Institute has individual and corporate members in over 90 countries. The aim of the Institute is to facilitate learning, recognise achievement, reward excellence and disseminate information within the global textiles, clothing and footwear industries. Historically, The Textile Institute has published books of interest to its members and the textile industry. To maintain this policy, the Institute has entered into partnership with Woodhead Publishing Limited to ensure that Institute members and the textile industry continue to have access to high calibre titles on textile science and technology. Most Woodhead titles on textiles are now published in collaboration with The Textile Institute. Through this arrangement, the Institute provides an Editorial Board which advises Woodhead on appropriate titles for future publication and suggests possible editors and authors for these books. Each book published under this arrangement carries the Institute’s logo. Woodhead books published in collaboration with The Textile Institute are offered to Textile Institute members at a substantial discount. These books, together with those published by The Textile Institute that are still in print, are offered on the Woodhead web site at: www.woodheadpublishing.com. Textile Institute books still in print are also available directly from the Institute’s website at: www.textileinstitutebooks.com. A list of Woodhead books on textile science and technology, most of which have been published in collaboration with The Textile Institute, can be found at the end of the contents pages. -

Bugs As Drugs, Part 1: Insects. the “New” Alternative Medicine for the 21St Century? E
amr Review Article Bugs as Drugs, Part 1: Insects. The “New” Alternative Medicine for the 21st Century? E. Paul Cherniack, MD Abstract estimates that $20 billion will be needed to replace Insects and insect-derived products have been widely used in the shortage of 800,000 conventional health care folk healing in many parts of the world since ancient times. workers by 2015.1 Globally ubiquitous, arthropods Promising treatments have at least preliminarily been studied potentially provide a cheap, plentiful supply of experimentally. Maggots and honey have been used to heal healing substances in an economically challenged chronic and post-surgical wounds and have been shown to be world. comparable to conventional dressings in numerous settings. Honey has also been applied to treat burns. Honey has been Maggots combined with beeswax in the care of several dermatologic The most well-studied medical application of disorders, including psoriasis, atopic dermatitis, tinea, arthropods is the use of maggots – the larvae of pityriasis versicolor, and diaper dermatitis. Royal jelly has flies (most frequently that of Lucilia sericata, a been used to treat postmenopausal symptoms. Bee and ant blowfly) that feed on necrotic tissue.2 Traditional venom have reduced the number of swollen joints in patients healers from many parts of the world including with rheumatoid arthritis. Propolis, a hive sealant made by Asia, South America, and Australia have used bees, has been utilized to cure aphthous stomatitis. “larval therapy,”3 and records of physician use of Cantharidin, a derivative of the bodies of blister beetles, has maggots to heal wounds have existed since the been applied to treat warts and molluscum contagiosum. -

An Initial in Vitro Investigation Into the Potential Therapeutic Use of Lucilia Sericata Maggot to Control Superficial Fungal Infections
Volume 6, Number 2, June .2013 ISSN 1995-6673 JJBS Pages 137 - 142 Jordan Journal of Biological Sciences An Initial In vitro Investigation into the Potential Therapeutic Use Of Lucilia sericata Maggot to Control Superficial Fungal Infections Sulaiman M. Alnaimat1,*, Milton Wainwright2 and Saleem H. Aladaileh 1 1 Biological Department, Al Hussein Bin Talal University, Ma’an, P.O. Box 20, Jordan; 2 Department of Molecular Biology and Biotechnology, University of Sheffield, Sheffield,S10 2TN, UK Received: November 12, 2012; accepted January 12, 2013 Abstract In this work an attempt was performed to investigate the in vitro ability of Lucilia sericata maggots to control fungi involved in superficial fungal infections. A novel GFP-modified yeast culture to enable direct visualization of the ingestion of yeast cells by maggot larvae as a method of control was used. The obtained results showed that the GFP-modified yeasts were successfully ingested by Lucilia sericata maggots and 1mg/ml of Lucilia sericata maggots excretions/ secretions (ES) showed a considerable anti-fungal activity against the growth of Trichophyton terrestre mycelium, the radial growth inhibition after 10 days of incubation reached 41.2 ±1.8 % in relation to the control, these results could lead to the possible application of maggot therapy in the treatment of wounds undergoing fungal infection. Keywords: Lucilia sericata, Maggot Therapy, Superficial Fungal Infections And Trichophyton Terrestre. (Sherman et al., 2000), including diabetic foot ulcers 1. Introduction (Sherman, 2003), malignant adenocarcinoma (Sealby, 2004), and for venous stasis ulcers (Sherman, 2009); it is Biosurgical debridement or "maggot therapy" is also used to combat infection after breast-conservation defined as the use of live, sterile maggots of certain type surgery (Church 2005). -

Debridement of Chronic Wounds: a Systematic Review
Health Technology Assessment 1999; Vol. 3: No. 17 (Pt 1) Review The debridement of chronic wounds: a systematic review M Bradley N Cullum T Sheldon Health Technology Assessment NHS R&D HTA Programme HTA HTA How to obtain copies of this and other HTA Programme reports. An electronic version of this publication, in Adobe Acrobat format, is available for downloading free of charge for personal use from the HTA website (http://www.hta.ac.uk). A fully searchable CD-ROM is also available (see below). Printed copies of HTA monographs cost £20 each (post and packing free in the UK) to both public and private sector purchasers from our Despatch Agents. Non-UK purchasers will have to pay a small fee for post and packing. For European countries the cost is £2 per monograph and for the rest of the world £3 per monograph. You can order HTA monographs from our Despatch Agents: – fax (with credit card or official purchase order) – post (with credit card or official purchase order or cheque) – phone during office hours (credit card only). Additionally the HTA website allows you either to pay securely by credit card or to print out your order and then post or fax it. Contact details are as follows: HTA Despatch Email: [email protected] c/o Direct Mail Works Ltd Tel: 02392 492 000 4 Oakwood Business Centre Fax: 02392 478 555 Downley, HAVANT PO9 2NP, UK Fax from outside the UK: +44 2392 478 555 NHS libraries can subscribe free of charge. Public libraries can subscribe at a very reduced cost of £100 for each volume (normally comprising 30–40 titles). -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

The Wound/Burn Guidelines –
doi: 10.1111/1346-8138.13288 Journal of Dermatology 2016; : 1–22 GUIDELINE The wound/burn guidelines – 6: Guidelines for the management of burns Yuichiro YOSHINO,1 Mikio OHTSUKA,2 Masakazu KAWAGUCHI,3 Keisuke SAKAI,4 Akira HASHIMOTO,5 Masahiro HAYASHI,3 Naoki MADOKORO,6 Yoshihide ASANO,7 Masatoshi ABE,8 Takayuki ISHII,9 Taiki ISEI,10 Takaaki ITO,11 Yuji INOUE,12 Shinichi IMAFUKU,13 Ryokichi IRISAWA,14 Masaki OHTSUKA,15 Fumihide OGAWA,16 Takafumi KADONO,7 Tamihiro KAWAKAMI,17 Ryuichi KUKINO,18 Takeshi KONO,19 Masanari KODERA,20 Masakazu TAKAHARA,21 Miki TANIOKA,22 Takeshi NAKANISHI,23 Yasuhiro NAKAMURA,24 Minoru HASEGAWA,9 Manabu FUJIMOTO,9 Hiroshi FUJIWARA,25 Takeo MAEKAWA,26 Koma MATSUO,27 Osamu YAMASAKI,15 Andres LE PAVOUX,28 Takao TACHIBANA,29 Hironobu IHN,12 The Wound/Burn Guidelines Committee 1Department of Dermatology, Japanese Red Cross Kumamoto Hospital, Kumamoto, 2Department of Dermatology, Fukushima Medical University, Fukushima, 3Department of Dermatology, Yamagata University Faculty of Medicine, Yamagata, 4Intensive Care Unit, Kumamoto University Hospital, Kumamoto, 5Department of Dermatology, Tohoku University Graduate School of Medicine, Miyagi, 6Department of Dermatology, Mazda Hospital, Hiroshima, 7Department of Dermatology, Faculty of Medicine,University of Tokyo, Tokyo, 8Department of Dermatology, Gunma University Graduate School of Medicine, Gunma, 9Department of Dermatology, Faculty of Medicine, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, Ishikawa, 10Department of Dermatology, Kansai -

Table 1B 2005 Community Prescription Numbers, Together with Government
TABLE 1B 2005 COMMUNITY PRESCRIPTION NUMBERS, TOGETHER WITH GOVERNMENT AND PATIENT COSTS FOR PBS-LISTED DRUGS Table 1B includes an estimate of community (non-public hospital) prescription numbers for the 2005 calendar year, costs (government and patient contribution) for the items with a four digit PBS/RPBS code, together with the defined daily dose (DDD) where assigned. There is no cost information available for items with a five digit Amfac drug code. Table 1 exclude the presentation of information on any item with an estimated community use of less than 110 prescriptions in 2005. The prescription items are arranged by ATC code on generic name, and by form and strength using either the PBS drug code (4 digit) or, for non-PBS items, the Amfac drug code (5 digit). Consult the index (page 255) by generic drug name to obtain the appropriate ATC code. An index by 2nd level of the ATC classification follows: ALIMENTARY TRACT AND METABOLISM PAGE NO A01 STOMATOLOGICAL PREPARATIONS 181 A02 DRUGS FOR ACID RELATED DISORDERS 182 A03 DRUGS FOR FUNCTIONAL GASTROINTESTINAL DISORDERS 185 A04 ANTIEMETICS AND ANTINAUSEANTS 187 A05 BILE AND LIVER THERAPY 188 A06 LAXATIVES 189 A07 ANTIDIARRHOEALS, INTESTINAL 191 ANTIINFLAMMATORY/ANTIINFECTIVE AGENTS A08 ANTIOBESITY PREPARATIONS, EXCLUDING DIET PRODUCTS 193 A09 DIGESTIVES, INCLUDING ENZYMES 194 A10 DRUGS USED IN DIABETES 195 A11 VITAMINS 197 A12 MINERAL SUPPLEMENTS 199 A14 ANABOLIC AGENTS FOR SYSTEMIC USE 200 177 BLOOD AND BLOOD FORMING ORGANS B01 ANTITHROMBOTIC AGENTS 201 B02 ANTIHAEMORRHAGICS 203 B03 -

Pharmacy Program and Drug Formulary
Pharmacy Program and Drug Formulary Secure Horizons Group Retiree Medicare Advantage Plan n Pharmacy Program Description n Platinum Plus Enhanced Formulary California Benefits Effective January 1, 2006 Table of Contents Your Secure Horizons Group Retiree Medicare Advantage Plan Prescription Drug Benefit........................................................................................................ iii Secure Horizons Pharmacy Program Definitions .................................................................... iii What Is the Platinum Plus Enhanced Formulary? ....................................................................iv Where to Have Your Prescriptions Filled .................................................................................iv Preferred and Non-Preferred Network Pharmacies .................................................................iv Network Preferred Pharmacy Locations ..................................................................................iv How to Fill a Prescription at a Network Pharmacy ...................................................................v Mail Service Pharmacy ..............................................................................................................v Secure Horizons Group Retiree Medicare Advantage Plan Offers a Two-Part Prescription Drug Benefit ..........................................................................vii Part 1 – Medicare Part D Prescription Drug Coverage ...................................................vii How Your Medicare -

Chronic Venous Ulcers: a Comparative Effectiveness Review of Treatment Modalities Comparative Effectiveness Review Number 127
Comparative Effectiveness Review Number 127 Chronic Venous Ulcers: A Comparative Effectiveness Review of Treatment Modalities Comparative Effectiveness Review Number 127 Chronic Venous Ulcers: A Comparative Effectiveness Review of Treatment Modalities Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Contract No. 290-2007-10061-I Prepared by: Johns Hopkins University Evidence-based Practice Center Baltimore, MD Investigators Jonathan Zenilman, M.D. M. Frances Valle, D.N.P., M.S. Mahmoud B. Malas, M.D., M.H.S. Nisa Maruthur, M.D., M.H.S. Umair Qazi, M.P.H. Yong Suh, M.B.A., M.Sc. Lisa M. Wilson, Sc.M. Elisabeth B. Haberl, B.A. Eric B. Bass, M.D., M.P.H. Gerald Lazarus, M.D. AHRQ Publication No. 13(14)-EHC121-EF December 2013 Erratum January 2014 This report is based on research conducted by the Johns Hopkins University Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 290-2007-10061-I). The findings and conclusions in this document are those of the author(s), who are responsible for its contents; the findings and conclusions do not necessarily represent the views of AHRQ. Therefore, no statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. The information in this report is intended to help health care decisionmakers—patients and clinicians, health system leaders, and policymakers, among others—make well-informed decisions and thereby improve the quality of health care services.