July 21, 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mouth Rinses
Mouth Rinses We may recommend a mouth rinse to you for one or more of the following reasons: Periodontal Disease, Gingivitis, Halitosis (bad breath), sensitive teeth, decay or increased risk of decay, dry mouth, minor mouth sore or irritation. Red= RX only Blue= Available through your dentist only Green= Over the counter *Common side effects found with many mouth rinses include the possibility of staining. We still encourage you to use the rinse that treats your problem because none of the stains these rinses cause are permanent. With good oral hygiene on your part, using your bleaching trays and bleach as needed, you can keep the staining to a minimum. If there is any staining that you can’t prevent, it can always be easily removed by your hygienist at your next cleaning. If you have periodontal disease or Gingivitis, we may recommend an antimicrobial/antiseptic type rinse. These rinses kill germs, reduce plaque, redness, swelling and bleeding when used in conjunction with proper brushing and flossing. Some common examples of antiseptic rinses are: Peridex or Perioguard oral rinses are available by prescription only. Active ingredient is chlorhexidine gluconate .12%. These also contain alcohol 11.6%. Can cause staining of the teeth*, taste alteration and dry mouth. Periomed Oral rinse is available through your dentist only. Active ingredient is stannous fluoride .63%. It is alcohol free. Periomed provides antimicrobial activity for up to 8 hours. Also promotes enamel remineralization, and helps reduce sensitivity. Can cause staining* and dry mouth. Listerine Antiseptic: Active ingredient is alcohol 26.9% in the original gold Listerine, and 21.6% in the other flavors. -

1 Brief Report: the Virucidal Efficacy of Oral Rinse Components Against SARS-Cov-2 in Vitro Evelina Statkute1†, Anzelika Rubin
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.13.381079; this version posted November 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Brief Report: The Virucidal Efficacy of Oral Rinse Components Against SARS-CoV-2 In Vitro Evelina Statkute1†, Anzelika Rubina1†, Valerie B O’Donnell1, David W. Thomas2† Richard J. Stanton1† 1Systems Immunity University Research Institute, Division of Infection & Immunity, School of Medicine, Heath Park, Cardiff, CF14 4XN 2Advanced Therapies Group, School of Dentistry, Cardiff University, Heath Park, Cardiff CF14 4XY, UK †These authors contributed equally * Correspondence: [email protected], [email protected] Running title: Virucidal Activity of Mouthwashes Keywords: SARS-CoV2, mouthwash, lipid, envelope Disclosure: Venture Life Group plc provided information on mouthwash formulations employed in the study, but had no role in funding, planning, execution, analysis or writing of this study. A separate study funded to Cardiff University by Venture Life Group is assessing in vivo efficacy of CPC in patients with COVID19. The investigators declare no direct conflicts exist. 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.11.13.381079; this version posted November 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. -
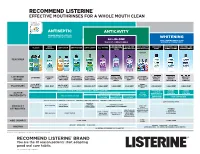
Listerine Floss Product Chart EN V9black
RECOMMEND LISTERINE® EFFECTIVE MOUTHRINSES FOR A WHOLE MOUTH CLEAN® 3 ANTISEPTIC ANTICAVITY 2X MORE HEALTHY SITES VS. JUST BRUSHING & FLOSSING WHITENING ALL-IN-ONE ONLY LEADING BRAND THAT THE MOST COMPLETE RINSE WHITENS & STRENGTHENS ALL-IN-ONE ZERO ALL-IN-ONE ANTI-CAVITY PEROXIDE WHITENS AND WHITENS AND CLASSIC ANTI-STAIN ANTI-TARTAR ANTI-CAVITY ALL-IN-ONE FOR SENSITIVE ALCOHOL TEETH ZERO ALCOHOL ZERO ALCOHOL FREE STRENGTHENS STRENGTHENS METERED DOSING FOR KIDS FEATURES ® LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE TOTAL CARE HEALTHY HEALTHY HEALTHY ULTRACLEAN ULTRACLEAN ULTRACLEAN TOTAL CARE SMART RINSE ZERO TOTAL CARE FOR SENSITIVE WHITE™ WHITE™ WHITE™ BRAND ANTI-STAIN ANTI-TARTAR ANTI-CAVITY TEETH ZERO FOR KIDS GENTLE RESTORING VIBRANT COOL MINT BERRY FRESHBURST ARCTIC MINT BUBBLE GUM FLAVOURS MILD MINT COOL MINT FRESHBURST CLEAN MINT CLEAN MINT MILD MINT CLEAN MINT CLEAN MINT CLEAN MINT ORIGINAL COOL CITRUS MINT EUCALYPTOL 0.091% W/V MENTHOL 0.042% W/V THYMOL 0.063% W/V SODIUM FLUORIDE SODIUM SODIUM 0.02% W/W FLUORIDE FLUORIDE ACTIVE SODIUM FLUORIDE 0.022% W/V SODIUM TETRAPOTASSIUM 0.022% W/V 0.02% W/W FLUORIDE PYROPHOSPHATE INGREDIENTS ZINC CHLORIDE 0.09% W/V ZINC CHLORIDE POTASSIUM ZINC CHLORIDE 0.022% W/V PENTASODIUM HYDROGEN PEROXIDE 0.09% W/V NITRATE 2.4% W/V 0.09% W/V TRIPHOSPHATE KILLS UP TO 99.9% OF GERMS IN YOUR MOUTH PREVENTS & REDUCES GINGIVITIS PREVENTS & REDUCES PLAQUE SAFELY WHITENS TEETH† PREVENTS CAVITIES MILD FLAVOUR HELPS KEEP PREVENTS CAVITIES PRODUCT TEETH WHITE STRENGTHENS STRENGTHENS TOOTH ENAMEL* 2X BETTER TOOTH ENAMEL CAVITY ATTRIBUTES PROTECTION VS. -
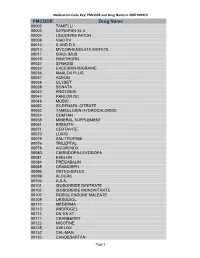
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS Cdc-Pdf
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00002 TAMIFLU 00003 DITROPAN XL II 00004 LIDODERM PATCH 00008 VIACTIV 00010 A AND D II 00013 MYCOPHENOLATE MOFETIL 00017 SIROLIMUS 00019 HAWTHORN 00027 SYNAGIS 00032 EXCEDRIN MIGRAINE 00036 MAALOX PLUS 00037 ACEON 00038 GLYSET 00039 SONATA 00042 PROTONIX 00044 PANLOR DC 00048 MOBIC 00052 SILDENAFIL CITRATE 00053 TAMSULOSIN HYDROCHLORIDE 00054 COMTAN 00058 MINERAL SUPPLEMENT 00061 BISMUTH 00071 CERTAVITE 00073 LUXIQ 00075 SAL-TROPINE 00076 TRILEPTAL 00078 AGGRENOX 00080 CARBIDOPA-LEVODOPA 00081 EXELON 00084 PREGABALIN 00085 ORAMORPH 00096 OSTEO-BIFLEX 00099 ALOCRIL 00100 A.S.A. 00101 ISOSORBIDE DINITRATE 00102 ISOSORBIDE MONONITRATE 00107 ROSIGLITAZONE MALEATE 00109 URSODIOL 00112 MEDERMA 00113 ANDROGEL 00114 DILTIA XT 00117 CRANBERRY 00123 NICOTINE 00125 AVELOX 00132 CAL-MAG 00133 CANDESARTAN Page 1 Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00148 PROLIXIN D 00149 D51/2 NS 00150 NICODERM CQ PATCH 00151 TUSSIN 00152 CEREZYME 00154 CHILDREN'S IBUPROFEN 00156 PROPOXACET-N 00159 KALETRA 00161 BISOPROLOL 00167 NOVOLIN N 00169 KETOROLAC TROMETHAMINE 00172 OPHTHALMIC OINTMENT 00173 ELA-MAX 00176 PREDNISOLONE ACETATE 00179 COLLOID SILVER 00184 KEPPRA 00187 OPHTHALMIC DROPS 00190 ABDEC 00191 HAPONAL 00192 SPECTRAVITE 00198 ENOXAPARIN SODIUM 00206 ACTONEL 00208 CELECOXIB 00209 GLUCOVANCE 00211 LEVALL 5.0 00213 PANTOPRAZOLE SODIUM 00217 TEMODAR 00218 CARBAMIDE PEROXIDE 00221 CHINESE HERBAL MEDS 00224 MILK AND MOLASSES ENEMA 00238 ZOLMITRIPTAN 00239 -

Milestone Guide and Workbook
Tulsa County Family Treatment Court Milestone Guide and Workbook 6th Edition May 2019 Tulsa County Family Treatment Court Table of Contents Milestone 0 Orientation Milestone 0 Checklist 3 Family Treatment Court Contract 4-6 Consent for Re-disclosure of Confidential Information 7 Medical Compliance Form 8 Family Treatment Court Countdown to Sobriety Worksheet 9 Drug Testing Program Requirements 10 Urine Analysis Collection Policy and Procedures 11 Courtroom Rules and Treatment Court Important Points to Remember 12 Client Information Sheet 13 Court Dress Code 14 Prescription Drug Policy 15-16 Doctors, Medicine, and Family Treatment Court Information 17 Approved Over the Counter Medication List 18 Milestone 1 White Belt Milestone 1 Checklist 19-20 Important Contact Information 21 Appointment Scheduler Worksheet 22 Rules for Mentees and Mentors 23-24 What are You Strengths? 25 Taking Care of Me 26 Appendix A 211 Information Packet Appendix B Narcotics Anonymous and Alcoholics Anonymous Meeting Information 2 Tulsa County Family Treatment Court Milestone 0 White Belt Orientation and Introduction Drug Test Color: Gold Completed orientation packet o Contract o Release of Confidential Information o Medical Compliance o Drug Testing Program Requirements o UA Collection o Client Information Sheet Received o Courtroom Rules o Dress Code o Prescription Drug Policy o Doctor, Medicine, and FTC info sheet Received White wrist band Issued FTC Guidebook binder Recognized to court. You starting court date is: Sober date: Your date to start drug testing is: The color line number is 918-596-8737 Drug testing is done at DATL o (2626 S. Sheridan, phone: 918-664-3285) 3 Tulsa County Family Treatment Court Family Treatment Court Contract I, _______________________________, have been accepted as a voluntary participant in the Tulsa County Family Treatment Court Program. -

Cosmetic Formulation of Skin Care Products.Pdf
DK9685_half-series-title 4/25/06 4:34 PM Page A Cosmetic Formulation of Skin Care Products DK9685_half-series-title 4/25/06 4:34 PM Page B COSMETIC SCIENCE AND TECHNOLOGY Series Editor ERIC JUNGERMANN Jungermann Associates, Inc. Phoenix, Arizona 1. Cosmetic and Drug Preservation: Principles and Practice, edited by Jon J. Kabara 2. The Cosmetic Industry: Scientific and Regulatory Foundations, edited by Norman F. Estrin 3. Cosmetic Product Testing: A Modern Psychophysical Approach, Howard R. Moskowitz 4. Cosmetic Analysis: Selective Methods and Techniques, edited by P. Boré 5. Cosmetic Safety: A Primer for Cosmetic Scientists, edited by James H. Whittam 6. Oral Hygiene Products and Practice, Morton Pader 7. Antiperspirants and Deodorants, edited by Karl Laden and Carl B. Felger 8. Clinical Safety and Efficacy Testing of Cosmetics, edited by William C. Waggoner 9. Methods for Cutaneous Investigation, edited by Robert L. Rietschel and Thomas S. Spencer 10. Sunscreens: Development, Evaluation, and Regulatory Aspects, edited by Nicholas J. Lowe and Nadim A. Shaath 11. Glycerine: A Key Cosmetic Ingredient, edited by Eric Jungermann and Norman O. V. Sonntag 12. Handbook of Cosmetic Microbiology, Donald S. Orth 13. Rheological Properties of Cosmetics and Toiletries, edited by Dennis Laba 14. Consumer Testing and Evaluation of Personal Care Products, Howard R. Moskowitz 15. Sunscreens: Development, Evaluation, and Regulatory Aspects. Second Edition, Revised and Expanded, edited by Nicholas J. Lowe, Nadim A. Shaath, and Madhu A. Pathak DK9685_half-series-title 4/25/06 4:34 PM Page C 16. Preservative-Free and Self-Preserving Cosmetics and Drugs: Principles and Practice, edited by Jon J. -

TITLE Safety and Efficacy of Over-The-Counter Drug Use by the Elderly
" DOCUMENT RESUME ED 245 148 CG 017 518 TITLE Safety and Efficacy of Over-the-Counter Drug Use by the Elderly. Hearing before the Subcommittee on Health and Long-Term Care of the Select Committee on Aging. House of Representatives, Ninety-Eighth Congress, First Session. INSTITUTION Congress of the U.S., Washington, D.C. House Select Committee on Aging. REPORT NO House-Comm-Pub-98-409 PUB DATE 21 Jul 83 NOTE 507p.; Some pages are marginally legible due to small print. PUB TYPE Legal/Legislative/Regulatory Materials (090) EDRS PRICE MF02 Plus Postage. PC Not Available from EDRS. DESCRIPTORS *Consumer Protection; Dietetics; Drug Legislation; *Drug Use; Gerontology; Hearings; *Older Adults; Physical Health; *Safety IDENTIFIERS Congress 98th; Long Term Care; *Nonprescription Drugs; *PPA ABSTRACT This document contains the prepared statements and panel testimony from the Congressional hearing on over-the-counter (OTC) drug use by the elderly. Opening statements are given by Representatives Claude Pepper (chairman), Ralph Regula, Mary Rose Oakar, Michael Bilirakis, Tom Lantos, and Hal Daub. Topics which are covered include the incidence and quantity of drug use by the elderly, health risks, adverse reactions, phenylpropanolamine (PPA), consumer protection, and the Federal Drug Administration's (FDA) role in the OTC drug safety and reguIatiem. Testimony of the first panel on OTC drugs, particularly weight reduction medications containing PPA, is given by representatives of the Health Research Group, the National Broadcasting Company, and consumers. Testimony of the second panel on mail fraud schemes perpetrated against senior citizens is given by consumer advocates representing the United States Postal Service, Criminal Investigations and Consumer Protection Divisions, and the Center for Science in the Public Interest. -

2019 SMDP Biotech Training Agenda & Scholar Bios
2019 SMDP Biotech 1-5 June PHILADELPHIA www.icpdprograms.org 2019 Scientist Mentoring & Diversity Program for biotechnology (SMDP Biotech) www.icpdprograms.org Training Session June 1-6, 2019 in Philadelphia, PA about the program who attends: the one-year career mentoring program pairs ethnically diverse post-baccalaureate students, graduate students and post-doctoral researchers with industry mentors who work at biotechnology and consumer healthcare companies. With their mentors, SMDP Biotech Scholars attend a 5-day training session to learn about career opportunities in industry and receive career coaching. SMDP Scholars and Mentors also attend The BIO International Convention. how to dress, what to bring: business attire with comfortable shoes. Scholars, bring 100 business cards and 10 copies of your resume. Mentors will need business cards too. Informal dinner where to go: SMDP training Scholars and Mentors you’re Host Hotel session day 1 already on the guest list, we Mentors you will arrange the bus leaves the leave J&J Consumer at 5:15pm your own accommodation host hotel at 6:30am Ladder 15, 1528 Sansom Street, Scholars a shared room Scholars you will be Philadelphia, PA 19102 reservation has already been introduced to your made for you at this location: Mentors at the Reading Terminal MarKet Philadelphia 201, 201 N. 17th training session The bus leaves from the SMDP J&J Consumer, Street, Philadelphia, PA 19103 training session at 11:30am 199 Grandview Road, Corner of North 12th Street “Celebration of Mentoring Skillman, NJ 08558 and Arch Street (South Bldg. SK1912) & Diversity” reception ($10 will be provided) (Saturday, June 1st at 6pm) SMDP training The BIO Intl Convention Scholars and Mentors you’re session day 2 registration information will be already on the guest list the bus leaves the provided during the SMDP The Pyramid Club, host hotel at 7am training session. -

Creative Director 561.714.1585 Andy Mathurin
Hello, I am: Looking for role as: Let’s Connect: Andy Mathurin Creative Director 561.714.1585 Associate Creative Director, [email protected] Brand Strategist New York What I do: PROFILE Brand Strategy Over 8 years leading branding and marketing concepts with career spanning 360 campaigns, broadcast, print, OOH, social, digital, video and experiential Identity Design across major worldwide brands. Energetic and a creative visionary offering Storytelling demonstrated expertise in all aspects of branding and strategy, with core focus Thought Leader on delivering business results. Team Builder Marketing WINS Project Management Successes have included winning several accounts for new business, resulting in agency being awarded Global Agency of the Year by AdAge & Adweek. Toolkit: AOR: Havas - Vascepa 2019, Havas - Alcon 2019, J3 - OGX 2018, UM - H&M 2017, UM - Sony 2017, BMW 2016, UM - Coca Cola 2016 Sketch XD Microsoft Office PROFESSIONAL EXPERIENCE Avocode 03.18 HAVAS | Manhattan, NY Photoshop Present Associate Creative Director Strategic partner, focusing on brand equity for healthcare and InDesign pharmaceutical brands. Responsible for new business and leading creative Illustration team/studio across all digital platforms (specializing in social). After Effects Brands include: Zicam, Alcon, GSK, NUCALA, TRELEGY, & ANORO. 09.14 Universal McCann Worldwide | Manhattan, NY 03.18 Personality: Associate Creative Director + Senior Art Director Hands-on leader in identity design. Champion participant in winning new Human business pitches. Guiding and advising clients on high-level executions. Confident Brands include: Sony Pictures, GoPro, McCormick, BMW... and more. Effective Communicator - Demonstrate strong leadership overseeing staff in the day-to-day projects Motivational Honest with Integrity - Deliver against demanding brand objectives and develop creative that Empathetic exceeds business needs, managing projects from concept through completion: timelines, budgets, schedules, etc. -

Propylene Glycol
PROPYLENE GLYCOL Your patch test result indicates that you have a contact allergy to propylene glycol. This contact allergy may cause your skin to react when it is exposed to this substance although it may take several days for the symptoms to appear. Typical symptoms include redness, swelling, itching, and fluid-filled blisters. Where is propylene glycol found? Propylene glycol is used as a softening agent, preservative, humectants, and solvent in cosmetics, fragrances, topical medications, soaps and cleansers, hair care products, and deodorants. Propylene glycol is also found in oral treatments as well as many foods. It is also added during the manufacture of many industrial fluids, such as solvents, thinners, antifreeze, other de-icing fluids, desiccants, brake fluids, and polyester resins. How can you avoid contact with propylene glycol? Avoid products that list any of the following names in the ingredients: • Propylene glycol • 1,2-Dihydroxypropane • CASRN: 57-55-6 • Methylethyl glycol • 1,2-Propanediol • 2-Hydroxypropanol • Isopropylene glycol What are some products that may contain propylene glycol? Antiperspirants and Deodorants: • Old Spice High Endurance • Meguiars Vinyl/Rubber Cleaner/Condition • Adidas 24 Hour Deodorant Control Antiperspirant & Deodorant • Pennzoil Roadside Fix A Flat Tire Sealant & • Adidas 24 Hour Fragrance Clear Stick • Old Spice High Endurance Deodorant Flat Preventative Deodorant • Old Spice Red Zone Clear Gel • Rain-X De-Icer (Aerosol) • Adidas Action 3 Tech F • Old Spice Red Zone Deodorant Stick • Slime -

Pg 1 of 189 Products and Pricing on the Alberta Blue Cross Drug Price List (ABCDPL) Effective April 13, 2017
Products and Pricing on the Alberta Blue Cross Drug Price List (ABCDPL) Effective April 13, 2017 DIN/ PIN/ NPN Product Description Route Form MFR Unit of Issue Base Price 00002192691 3TC 10 MG/ML ORAL LIQUID ORL LIQ GSK ML 0.3307 00002192683 3TC 150 MG TABLET ORL TAB GSK TAB 5.0507 00002247825 3TC 300 MG TABLET ORL TAB GSK TAB 9.8070 00002162113 5 BENZAGEL 5% TOPICAL (ALCOHOL) GEL TOP ALCGEL CLC G 0.2080 00002166607 5 BENZAGEL 5% TOPICAL LOTION TOP LOT NVC ML 0.1984 00000437999 5% DEXTROSE & 0.45% NACL W 0.15% KCL 20 MEQ INJ INJ DEFAUL BAX ML 0.0030 00000037974 50% DEXTROSE 500 MG/ML INJECTION USP INJ DEFAUL HSP ML 0.5842 00080013172 5-HTP 100 MG CAPSULE ORL CAP VTH CAP 0.2000 00002414570 ABBOTT-CITALOPRAM 10 MG TABLET ORL TAB ABB TAB 0.1432 00002414589 ABBOTT-CITALOPRAM 20 MG TABLET ORL TAB ABB TAB 0.2397 00002414597 ABBOTT-CITALOPRAM 40 MG TABLET ORL TAB ABB TAB 0.2397 00002412942 ABBOTT-CLOPIDOGREL 75 MG TABLET ORL TAB ABB TAB 0.4735 00002414805 ABBOTT-LEVETIRACETAM 250 MG TABLET ORL TAB ABB TAB 0.8000 00002414791 ABBOTT-LEVETIRACETAM 500 MG TABLET ORL TAB ABB TAB 0.9750 00002414783 ABBOTT-LEVETIRACETAM 750 MG TABLET ORL TAB ABB TAB 1.3500 00002414546 ABBOTT-OLANZAPINE ODT 10 MG TABLET ORL DISNTAB ABB TAB 1.2857 00002414538 ABBOTT-OLANZAPINE ODT 5 MG TABLET ORL DISNTAB ABB TAB 0.6434 00002412969 ABBOTT-PANTOPRAZOLE 40 MG ENTERIC-COATED TABLET ORL ECT ABB TAB 0.3628 00002412985 ABBOTT-QUETIAPINE 100 MG TABLET ORL TAB ABB TAB 0.3295 00002412993 ABBOTT-QUETIAPINE 200 MG TABLET ORL TAB ABB TAB 0.6617 00002412977 ABBOTT-QUETIAPINE 25 MG TABLET ORL TAB ABB TAB 0.1235 00002413000 ABBOTT-QUETIAPINE 300 MG TABLET ORL TAB ABB TAB 0.9656 00002422638 ABBOTT-RABEPRAZOLE 10 MG ENTERIC-COATED TABLET ORL ECT ABB TAB 0.1204 00002422646 ABBOTT-RABEPRAZOLE 20 MG ENTERIC-COATED TABLET ORL ECT ABB TAB 0.2408 00002414619 ABBOTT-TOPIRAMATE 100 MG TABLET ORL TAB ABB TAB 0.5928 This document is for pricing use only and should not be used to determine benefit status information. -

Caries Risk Assessment
OCTOBER CAMBRA Clinical Protocols Journal Products - Caries risk assessment Douglas A. Young, DDS, MS, MBA; John D.B. Featherstone, MSc, PhD; and Jon R. Roth, MS, CAE CDA Journal Volume 35, Number 10 Journal october 2007 departments 665 The Editor/Health Illiteracy 667 Letters to the Editor 671 Impressions 754 Dr. Bob/Dental Spa-ahhhhh features 679 CARIES MANAGEMENT BY RISK ASSESSMENT — A PRACtitioner’S GUIDE An introduction to the issue. Douglas A. Young, DDS, MS, MBA; John D.B. Featherstone, MSc, PhD; and Jon R. Roth, MS, CAE 681 CURING THE SILENT EPIDEMIC: CARIES MANAGEMENT IN the 21sT CENTURY AND BEYOND This paper will present key concepts necessary for the most current management of dental caries and sets the stage for subsequent papers in this issue to cover the clinical implementation of a caries management by risk assessment model (CAMBRA). Douglas A. Young, DDS, MS, MBA; John D.B. Featherstone, MSc, PhD; and Jon R. Roth, MS, CAE 687 CARIES RISK ASSESSMENT APPROPRIATE FOR THE Age 1 VISIT (INFANTS AND Toddlers) The latest maternal and child Caries Management By Risk Assessment tools for children age 0 to 5 (CAMBRA 0-5), developed for oral health promotion and disease prevention starting with the recommended age 1 dental visit is presented in this paper. Francisco J. Ramos-Gomez, DDS, MS, MPH; James Crall, DDS, ScD; Stuart A. Gansky, DrPH; Rebecca L. Slayton, DDS, PhD; and John D.B. Featherstone, MSc, PhD 703 CARIES RISK ASSESSMENT IN PRACTICE FOR Age 6 THROUGH ADULT A practical caries risk assessment procedure and form for patients age 6 through adult are presented.