Milestone Guide and Workbook
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mouth Rinses
Mouth Rinses We may recommend a mouth rinse to you for one or more of the following reasons: Periodontal Disease, Gingivitis, Halitosis (bad breath), sensitive teeth, decay or increased risk of decay, dry mouth, minor mouth sore or irritation. Red= RX only Blue= Available through your dentist only Green= Over the counter *Common side effects found with many mouth rinses include the possibility of staining. We still encourage you to use the rinse that treats your problem because none of the stains these rinses cause are permanent. With good oral hygiene on your part, using your bleaching trays and bleach as needed, you can keep the staining to a minimum. If there is any staining that you can’t prevent, it can always be easily removed by your hygienist at your next cleaning. If you have periodontal disease or Gingivitis, we may recommend an antimicrobial/antiseptic type rinse. These rinses kill germs, reduce plaque, redness, swelling and bleeding when used in conjunction with proper brushing and flossing. Some common examples of antiseptic rinses are: Peridex or Perioguard oral rinses are available by prescription only. Active ingredient is chlorhexidine gluconate .12%. These also contain alcohol 11.6%. Can cause staining of the teeth*, taste alteration and dry mouth. Periomed Oral rinse is available through your dentist only. Active ingredient is stannous fluoride .63%. It is alcohol free. Periomed provides antimicrobial activity for up to 8 hours. Also promotes enamel remineralization, and helps reduce sensitivity. Can cause staining* and dry mouth. Listerine Antiseptic: Active ingredient is alcohol 26.9% in the original gold Listerine, and 21.6% in the other flavors. -

1 Brief Report: the Virucidal Efficacy of Oral Rinse Components Against SARS-Cov-2 in Vitro Evelina Statkute1†, Anzelika Rubin
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.13.381079; this version posted November 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Brief Report: The Virucidal Efficacy of Oral Rinse Components Against SARS-CoV-2 In Vitro Evelina Statkute1†, Anzelika Rubina1†, Valerie B O’Donnell1, David W. Thomas2† Richard J. Stanton1† 1Systems Immunity University Research Institute, Division of Infection & Immunity, School of Medicine, Heath Park, Cardiff, CF14 4XN 2Advanced Therapies Group, School of Dentistry, Cardiff University, Heath Park, Cardiff CF14 4XY, UK †These authors contributed equally * Correspondence: [email protected], [email protected] Running title: Virucidal Activity of Mouthwashes Keywords: SARS-CoV2, mouthwash, lipid, envelope Disclosure: Venture Life Group plc provided information on mouthwash formulations employed in the study, but had no role in funding, planning, execution, analysis or writing of this study. A separate study funded to Cardiff University by Venture Life Group is assessing in vivo efficacy of CPC in patients with COVID19. The investigators declare no direct conflicts exist. 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.11.13.381079; this version posted November 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. -
Listerine Floss Product Chart EN V9black
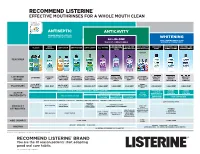
RECOMMEND LISTERINE® EFFECTIVE MOUTHRINSES FOR A WHOLE MOUTH CLEAN® 3 ANTISEPTIC ANTICAVITY 2X MORE HEALTHY SITES VS. JUST BRUSHING & FLOSSING WHITENING ALL-IN-ONE ONLY LEADING BRAND THAT THE MOST COMPLETE RINSE WHITENS & STRENGTHENS ALL-IN-ONE ZERO ALL-IN-ONE ANTI-CAVITY PEROXIDE WHITENS AND WHITENS AND CLASSIC ANTI-STAIN ANTI-TARTAR ANTI-CAVITY ALL-IN-ONE FOR SENSITIVE ALCOHOL TEETH ZERO ALCOHOL ZERO ALCOHOL FREE STRENGTHENS STRENGTHENS METERED DOSING FOR KIDS FEATURES ® LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE TOTAL CARE HEALTHY HEALTHY HEALTHY ULTRACLEAN ULTRACLEAN ULTRACLEAN TOTAL CARE SMART RINSE ZERO TOTAL CARE FOR SENSITIVE WHITE™ WHITE™ WHITE™ BRAND ANTI-STAIN ANTI-TARTAR ANTI-CAVITY TEETH ZERO FOR KIDS GENTLE RESTORING VIBRANT COOL MINT BERRY FRESHBURST ARCTIC MINT BUBBLE GUM FLAVOURS MILD MINT COOL MINT FRESHBURST CLEAN MINT CLEAN MINT MILD MINT CLEAN MINT CLEAN MINT CLEAN MINT ORIGINAL COOL CITRUS MINT EUCALYPTOL 0.091% W/V MENTHOL 0.042% W/V THYMOL 0.063% W/V SODIUM FLUORIDE SODIUM SODIUM 0.02% W/W FLUORIDE FLUORIDE ACTIVE SODIUM FLUORIDE 0.022% W/V SODIUM TETRAPOTASSIUM 0.022% W/V 0.02% W/W FLUORIDE PYROPHOSPHATE INGREDIENTS ZINC CHLORIDE 0.09% W/V ZINC CHLORIDE POTASSIUM ZINC CHLORIDE 0.022% W/V PENTASODIUM HYDROGEN PEROXIDE 0.09% W/V NITRATE 2.4% W/V 0.09% W/V TRIPHOSPHATE KILLS UP TO 99.9% OF GERMS IN YOUR MOUTH PREVENTS & REDUCES GINGIVITIS PREVENTS & REDUCES PLAQUE SAFELY WHITENS TEETH† PREVENTS CAVITIES MILD FLAVOUR HELPS KEEP PREVENTS CAVITIES PRODUCT TEETH WHITE STRENGTHENS STRENGTHENS TOOTH ENAMEL* 2X BETTER TOOTH ENAMEL CAVITY ATTRIBUTES PROTECTION VS. -

July 21, 2021
1 2nd Quarter 2021 Earnings Call July 21, 2021 Cautionary Note on Forward-looking Statements This presentation contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things: future operating and financial performance, product development, market position and business strategy. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to: risks related to the impact of the COVID-19 global pandemic, such as the scope and duration of the outbreak, government actions and restrictive measures implemented in response, material delays and cancellations of medical procedures, supply chain disruptions and other impacts to the business, or on the Company’s ability to execute business continuity plans, as a result of the COVID-19 pandemic; economic factors, such as interest rate and currency exchange rate fluctuations; competition, including technological advances, new products and patents attained by competitors; challenges inherent in new product research and development, including uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new and existing products; challenges to patents; the impact -

TITLE Safety and Efficacy of Over-The-Counter Drug Use by the Elderly
" DOCUMENT RESUME ED 245 148 CG 017 518 TITLE Safety and Efficacy of Over-the-Counter Drug Use by the Elderly. Hearing before the Subcommittee on Health and Long-Term Care of the Select Committee on Aging. House of Representatives, Ninety-Eighth Congress, First Session. INSTITUTION Congress of the U.S., Washington, D.C. House Select Committee on Aging. REPORT NO House-Comm-Pub-98-409 PUB DATE 21 Jul 83 NOTE 507p.; Some pages are marginally legible due to small print. PUB TYPE Legal/Legislative/Regulatory Materials (090) EDRS PRICE MF02 Plus Postage. PC Not Available from EDRS. DESCRIPTORS *Consumer Protection; Dietetics; Drug Legislation; *Drug Use; Gerontology; Hearings; *Older Adults; Physical Health; *Safety IDENTIFIERS Congress 98th; Long Term Care; *Nonprescription Drugs; *PPA ABSTRACT This document contains the prepared statements and panel testimony from the Congressional hearing on over-the-counter (OTC) drug use by the elderly. Opening statements are given by Representatives Claude Pepper (chairman), Ralph Regula, Mary Rose Oakar, Michael Bilirakis, Tom Lantos, and Hal Daub. Topics which are covered include the incidence and quantity of drug use by the elderly, health risks, adverse reactions, phenylpropanolamine (PPA), consumer protection, and the Federal Drug Administration's (FDA) role in the OTC drug safety and reguIatiem. Testimony of the first panel on OTC drugs, particularly weight reduction medications containing PPA, is given by representatives of the Health Research Group, the National Broadcasting Company, and consumers. Testimony of the second panel on mail fraud schemes perpetrated against senior citizens is given by consumer advocates representing the United States Postal Service, Criminal Investigations and Consumer Protection Divisions, and the Center for Science in the Public Interest. -

2019 SMDP Biotech Training Agenda & Scholar Bios
2019 SMDP Biotech 1-5 June PHILADELPHIA www.icpdprograms.org 2019 Scientist Mentoring & Diversity Program for biotechnology (SMDP Biotech) www.icpdprograms.org Training Session June 1-6, 2019 in Philadelphia, PA about the program who attends: the one-year career mentoring program pairs ethnically diverse post-baccalaureate students, graduate students and post-doctoral researchers with industry mentors who work at biotechnology and consumer healthcare companies. With their mentors, SMDP Biotech Scholars attend a 5-day training session to learn about career opportunities in industry and receive career coaching. SMDP Scholars and Mentors also attend The BIO International Convention. how to dress, what to bring: business attire with comfortable shoes. Scholars, bring 100 business cards and 10 copies of your resume. Mentors will need business cards too. Informal dinner where to go: SMDP training Scholars and Mentors you’re Host Hotel session day 1 already on the guest list, we Mentors you will arrange the bus leaves the leave J&J Consumer at 5:15pm your own accommodation host hotel at 6:30am Ladder 15, 1528 Sansom Street, Scholars a shared room Scholars you will be Philadelphia, PA 19102 reservation has already been introduced to your made for you at this location: Mentors at the Reading Terminal MarKet Philadelphia 201, 201 N. 17th training session The bus leaves from the SMDP J&J Consumer, Street, Philadelphia, PA 19103 training session at 11:30am 199 Grandview Road, Corner of North 12th Street “Celebration of Mentoring Skillman, NJ 08558 and Arch Street (South Bldg. SK1912) & Diversity” reception ($10 will be provided) (Saturday, June 1st at 6pm) SMDP training The BIO Intl Convention Scholars and Mentors you’re session day 2 registration information will be already on the guest list the bus leaves the provided during the SMDP The Pyramid Club, host hotel at 7am training session. -

Creative Director 561.714.1585 Andy Mathurin
Hello, I am: Looking for role as: Let’s Connect: Andy Mathurin Creative Director 561.714.1585 Associate Creative Director, [email protected] Brand Strategist New York What I do: PROFILE Brand Strategy Over 8 years leading branding and marketing concepts with career spanning 360 campaigns, broadcast, print, OOH, social, digital, video and experiential Identity Design across major worldwide brands. Energetic and a creative visionary offering Storytelling demonstrated expertise in all aspects of branding and strategy, with core focus Thought Leader on delivering business results. Team Builder Marketing WINS Project Management Successes have included winning several accounts for new business, resulting in agency being awarded Global Agency of the Year by AdAge & Adweek. Toolkit: AOR: Havas - Vascepa 2019, Havas - Alcon 2019, J3 - OGX 2018, UM - H&M 2017, UM - Sony 2017, BMW 2016, UM - Coca Cola 2016 Sketch XD Microsoft Office PROFESSIONAL EXPERIENCE Avocode 03.18 HAVAS | Manhattan, NY Photoshop Present Associate Creative Director Strategic partner, focusing on brand equity for healthcare and InDesign pharmaceutical brands. Responsible for new business and leading creative Illustration team/studio across all digital platforms (specializing in social). After Effects Brands include: Zicam, Alcon, GSK, NUCALA, TRELEGY, & ANORO. 09.14 Universal McCann Worldwide | Manhattan, NY 03.18 Personality: Associate Creative Director + Senior Art Director Hands-on leader in identity design. Champion participant in winning new Human business pitches. Guiding and advising clients on high-level executions. Confident Brands include: Sony Pictures, GoPro, McCormick, BMW... and more. Effective Communicator - Demonstrate strong leadership overseeing staff in the day-to-day projects Motivational Honest with Integrity - Deliver against demanding brand objectives and develop creative that Empathetic exceeds business needs, managing projects from concept through completion: timelines, budgets, schedules, etc. -

Propylene Glycol
PROPYLENE GLYCOL Your patch test result indicates that you have a contact allergy to propylene glycol. This contact allergy may cause your skin to react when it is exposed to this substance although it may take several days for the symptoms to appear. Typical symptoms include redness, swelling, itching, and fluid-filled blisters. Where is propylene glycol found? Propylene glycol is used as a softening agent, preservative, humectants, and solvent in cosmetics, fragrances, topical medications, soaps and cleansers, hair care products, and deodorants. Propylene glycol is also found in oral treatments as well as many foods. It is also added during the manufacture of many industrial fluids, such as solvents, thinners, antifreeze, other de-icing fluids, desiccants, brake fluids, and polyester resins. How can you avoid contact with propylene glycol? Avoid products that list any of the following names in the ingredients: • Propylene glycol • 1,2-Dihydroxypropane • CASRN: 57-55-6 • Methylethyl glycol • 1,2-Propanediol • 2-Hydroxypropanol • Isopropylene glycol What are some products that may contain propylene glycol? Antiperspirants and Deodorants: • Old Spice High Endurance • Meguiars Vinyl/Rubber Cleaner/Condition • Adidas 24 Hour Deodorant Control Antiperspirant & Deodorant • Pennzoil Roadside Fix A Flat Tire Sealant & • Adidas 24 Hour Fragrance Clear Stick • Old Spice High Endurance Deodorant Flat Preventative Deodorant • Old Spice Red Zone Clear Gel • Rain-X De-Icer (Aerosol) • Adidas Action 3 Tech F • Old Spice Red Zone Deodorant Stick • Slime -

Caries Risk Assessment
OCTOBER CAMBRA Clinical Protocols Journal Products - Caries risk assessment Douglas A. Young, DDS, MS, MBA; John D.B. Featherstone, MSc, PhD; and Jon R. Roth, MS, CAE CDA Journal Volume 35, Number 10 Journal october 2007 departments 665 The Editor/Health Illiteracy 667 Letters to the Editor 671 Impressions 754 Dr. Bob/Dental Spa-ahhhhh features 679 CARIES MANAGEMENT BY RISK ASSESSMENT — A PRACtitioner’S GUIDE An introduction to the issue. Douglas A. Young, DDS, MS, MBA; John D.B. Featherstone, MSc, PhD; and Jon R. Roth, MS, CAE 681 CURING THE SILENT EPIDEMIC: CARIES MANAGEMENT IN the 21sT CENTURY AND BEYOND This paper will present key concepts necessary for the most current management of dental caries and sets the stage for subsequent papers in this issue to cover the clinical implementation of a caries management by risk assessment model (CAMBRA). Douglas A. Young, DDS, MS, MBA; John D.B. Featherstone, MSc, PhD; and Jon R. Roth, MS, CAE 687 CARIES RISK ASSESSMENT APPROPRIATE FOR THE Age 1 VISIT (INFANTS AND Toddlers) The latest maternal and child Caries Management By Risk Assessment tools for children age 0 to 5 (CAMBRA 0-5), developed for oral health promotion and disease prevention starting with the recommended age 1 dental visit is presented in this paper. Francisco J. Ramos-Gomez, DDS, MS, MPH; James Crall, DDS, ScD; Stuart A. Gansky, DrPH; Rebecca L. Slayton, DDS, PhD; and John D.B. Featherstone, MSc, PhD 703 CARIES RISK ASSESSMENT IN PRACTICE FOR Age 6 THROUGH ADULT A practical caries risk assessment procedure and form for patients age 6 through adult are presented. -

Lifescan Ethicon
LouiseLouise MehrotraMehrotra ViceVice PresidentPresident InvestorInvestor RelationsRelations ““SafeSafe HarborHarbor”” StatementStatement This presentation may contain “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could vary materially from the Company’s expectations and projections. Risks and uncertainties include general industry conditions and competition; economic conditions, such as interest rate and currency exchange rate fluctuations; technological advances and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approvals; domestic and foreign health care reforms and governmental laws and regulations; and trends toward health care cost containment. A further list and description of these risks, uncertainties and other factors can be found in Exhibit 99 to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2006. Copies of this Form 10-K, as well as subsequent filings, are available online at www.sec.gov, www.jnj.com or on request from the Company. The Company does not undertake to update any forward-looking statements as a result of new information or future events or developments. WilliamWilliam C.C. WeldonWeldon ChairmanChairman ofof thethe BoardBoard && ChiefChief ExecutiveExecutive OfficerOfficer TodayToday’’ss AgendaAgenda -

Annual Report
ANNUAL REPORT 2019 MARCH 2020 To Our Shareholders Alex Gorsky Chairman and Chief Executive Officer By just about every measure, Johnson & These are some of the many financial and Johnson’s 133rd year was extraordinary. strategic achievements that were made possible by the commitment of our more than • We delivered strong operational revenue and 132,000 Johnson & Johnson colleagues, who adjusted operational earnings growth* that passionately lead the way in improving the health exceeded the financial performance goals we and well-being of people around the world. set for the Company at the start of 2019. • We again made record investments in research and development (R&D)—more than $11 billion across our Pharmaceutical, Medical Devices Propelled by our people, products, and and Consumer businesses—as we maintained a purpose, we look forward to the future relentless pursuit of innovation to develop vital with great confidence and optimism scientific breakthroughs. as we remain committed to leading • We proudly launched new transformational across the spectrum of healthcare. medicines for untreated and treatment-resistant diseases, while gaining approvals for new uses of many of our medicines already in the market. Through proactive leadership across our enterprise, we navigated a constant surge • We deployed approximately $7 billion, of unique and complex challenges, spanning primarily in transactions that fortify our dynamic global issues, shifting political commitment to digital surgery for a more climates, industry and competitive headwinds, personalized and elevated standard of and an ongoing litigious environment. healthcare, and that enhance our position in consumer skin health. As we have experienced for 133 years, we • And our teams around the world continued can be sure that 2020 will present a new set of working to address pressing public health opportunities and challenges. -

The Potential Benefit of Mouthwashes in Reducing COVID-19 Viral Load: a Mini Review
REVIEW ARTICLE The Potential Benefit of Mouthwashes in Reducing COVID-19 Viral Load: A Mini Review Olugbenga S. Oyeniyi1, Foluke Bosun-Arije2, Rotimi A. K. Jaiyesimi3* Affiliation 1Faculty of Health Sciences and Wellbeing, University of Sunderland, UK; 2Faculty of Health, Psychology and Social Care, Manchester Metropolitan University, UK; 3Associate Medical Director for Patient Safety & Consultant Obstetrician and Gynaecologist, Basildon and Thurrock University Hospital, UK. *Corresponding Author: Rotimi A. K. Jaiyesimi Email: [email protected]; Tel: +447415900457 Abstract Scientists throughout the world are searching for a lasting solution in the form of a vaccine or a drug that could be used to combat COVID-19 infections. A number of studies have proposed the antiviral efficacy of mouthwashes across a different population. Research has shown over the years that active ingredients present in commercially available mouthwash are potent enough to damage viruses, particularly those with the lipid envelope, rendering them harmless. This paper reviews the effect of mouthwashes on few viruses, including those possibly linked to SARS-COV-2. The aim is to provide evidence on the potential benefit of mouthwashes in reducing viral load and pool together the impact of various mouthwashes in managing individuals diagnosed with viral infections. We searched academic, English- scripted paper published between 1995 and June 2020 on PubMed, MEDLINE, ScienceDirect, EMBASE, WHO and the Cochrane Library databases. Two review authors independently assessed the eligibility and quality of the retrieved papers using the Jadad scale. Result showed there were evidences indicating that the benefits of mouthwashes to viral infections might be transferrable to COVID-19. However, future trials are recommended to establish the benefits of mouthwashes in reducing the burden of COVID-19.