2019 GLOBAL WINNER Videos Produced By
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Benadryl Allergy Relief Plus Decongestant Capsules
Distance between cutted line/middel of the spot = 51.6 mm spot = 12 x 2 centered in the margin of 14 mm le f 14 mm GB Benadryl Allergy Relief Plus Decon Caps 12s PIL MAH Update CRD 6738 t margin 23.06.2021 English 402428175_r0 N/A 1 Brochure/Leaet/Insert 145 x 250 mm Capsules are a medicine which are used to blood pressure). 364350C IM_Val-de-Reuil_Upper Normandy_France relieve the symptoms of hay fever and similar ■ If you have glaucoma (increased pressure allergic conditions. The capsules contain in the eye). CTE LITE 364350B Westrock Saint Pierre des Corps pseudoephedrine hydrochloride, which is a ■ If you have an overactive thyroid gland. 845 Oset Printing decongestant that relieves nasal and sinus ■ If you have severe kidney problems. 7996903 PAPER congestion and acrivastine which is an ■ If you are taking, or have taken in the last EMEA_2021_00024796_003 PC-0002778 antihistamine that helps relieve allergy two weeks, drugs for depression known symptoms such as sneezing, runny nose and as Monoamine Oxidase Inhibitors CAPSULES watery eyes. (MAOIs) or Reverse Inhibitors of Acrivastine 8mg & Pseudoephedrine 60mg Monoamine Oxidase 2 Before taking this (RIMAs). ■ This medicine is used to relieve the symptoms ■ If you are taking any cough or of hay fever and similar allergic conditions. cold medicines. PANTONE PANTONE medicine 356 C 287 C ■ This medicine is for use by adults and If any of these apply to you, get advice from children aged 12 - 65 years. This medicine is suitable for most adults under LITHO OFFSET LITHO OFFSET 65 years old and children aged 12 years and a doctor or pharmacist without taking ■ Do not take this medicine: REPRESENTATION: Colours represented with a diagonal line have been modified to aid PDF approval. -

Learner Notification International Society for Heart & Lung
Learner Notification International Society for Heart & Lung Transplantation (ISHLT) 41st Annual Meeting & Scientific Sessions Virtual Experience April 24 – 28, 2021 Live Virtual Acknowledgement of Financial Commercial Support Abbott Medtronic United Therapeutics Acknowledgement of In-Kind Commercial Support No in-kind commercial support was received for this educational activity. Satisfactory Completion Learners must complete an evaluation form to receive a certificate of completion. Your chosen sessions must be attended in their entirety as partial credit of individual sessions is not available. If you are seeking continuing education credit for a specialty not listed below, it is your responsibility to contact your licensing/certification board to determine course eligibility for your licensing/certification requirement. Physicians (ACCME) The International Society for Heart and Lung Transplantation (ISHLT) is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. Credit Designation Statement - ISHLT designates this live virtual activity for a maximum of 32.00 AMA PRA Category 1 CreditsTM. Physicians should claim only the credit commensurate with the extent of their participation in the activity. Accreditation Statement In support of improving patient care, this activity has been planned and implemented by Amedco LLC and ISHLT. Amedco LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. Nurses (ANCC) - Credit Designation Statement - Amedco LLC designates this live virtual activity for a maximum of 32.00 ANCC contact hours. Pharmacists (ACPE) - Credit Designation Statement - Amedco LLC designates this live virtual activity for a maximum of 32.00 knowledge-based CPE contact hours. -

Mouth Rinses
Mouth Rinses We may recommend a mouth rinse to you for one or more of the following reasons: Periodontal Disease, Gingivitis, Halitosis (bad breath), sensitive teeth, decay or increased risk of decay, dry mouth, minor mouth sore or irritation. Red= RX only Blue= Available through your dentist only Green= Over the counter *Common side effects found with many mouth rinses include the possibility of staining. We still encourage you to use the rinse that treats your problem because none of the stains these rinses cause are permanent. With good oral hygiene on your part, using your bleaching trays and bleach as needed, you can keep the staining to a minimum. If there is any staining that you can’t prevent, it can always be easily removed by your hygienist at your next cleaning. If you have periodontal disease or Gingivitis, we may recommend an antimicrobial/antiseptic type rinse. These rinses kill germs, reduce plaque, redness, swelling and bleeding when used in conjunction with proper brushing and flossing. Some common examples of antiseptic rinses are: Peridex or Perioguard oral rinses are available by prescription only. Active ingredient is chlorhexidine gluconate .12%. These also contain alcohol 11.6%. Can cause staining of the teeth*, taste alteration and dry mouth. Periomed Oral rinse is available through your dentist only. Active ingredient is stannous fluoride .63%. It is alcohol free. Periomed provides antimicrobial activity for up to 8 hours. Also promotes enamel remineralization, and helps reduce sensitivity. Can cause staining* and dry mouth. Listerine Antiseptic: Active ingredient is alcohol 26.9% in the original gold Listerine, and 21.6% in the other flavors. -

2015 Annual Report
ANNUAL REPORT 2015 MARCH 2016 TO OUR SHAREHOLDERS ALEX GORSKY Chairman, Board of Directors and Chief Executive Officer This year at Johnson & Johnson, we are proud this aligned with our values. Our Board of WRITTEN OVER to celebrate 130 years of helping people Directors engages in a formal review of 70 YEARS AGO, everywhere live longer, healthier and happier our strategic plans, and provides regular OUR CREDO lives. As I reflect on our heritage and consider guidance to ensure our strategy will continue UNITES & our future, I am optimistic and confident in the creating better outcomes for the patients INSPIRES THE long-term potential for our business. and customers we serve, while also creating EMPLOYEES long-term value for our shareholders. OF JOHNSON We manage our business using a strategic & JOHNSON. framework that begins with Our Credo. Written OUR STRATEGIES ARE BASED ON over 70 years ago, it unites and inspires the OUR BROAD AND DEEP KNOWLEDGE employees of Johnson & Johnson. It reminds OF THE HEALTH CARE LANDSCAPE us that our first responsibility is to the patients, IN WHICH WE OPERATE. customers and health care professionals who For 130 years, our company has been use our products, and it compels us to deliver driving breakthrough innovation in health on our responsibilities to our employees, care – from revolutionizing wound care in communities and shareholders. the 1880s to developing cures, vaccines and treatments for some of today’s most Our strategic framework positions us well pressing diseases in the world. We are acutely to continue our leadership in the markets in aware of the need to evaluate our business which we compete through a set of strategic against the changing health care environment principles: we are broadly based in human and to challenge ourselves based on the health care, our focus is on managing for the results we deliver. -

1 Brief Report: the Virucidal Efficacy of Oral Rinse Components Against SARS-Cov-2 in Vitro Evelina Statkute1†, Anzelika Rubin
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.13.381079; this version posted November 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Brief Report: The Virucidal Efficacy of Oral Rinse Components Against SARS-CoV-2 In Vitro Evelina Statkute1†, Anzelika Rubina1†, Valerie B O’Donnell1, David W. Thomas2† Richard J. Stanton1† 1Systems Immunity University Research Institute, Division of Infection & Immunity, School of Medicine, Heath Park, Cardiff, CF14 4XN 2Advanced Therapies Group, School of Dentistry, Cardiff University, Heath Park, Cardiff CF14 4XY, UK †These authors contributed equally * Correspondence: [email protected], [email protected] Running title: Virucidal Activity of Mouthwashes Keywords: SARS-CoV2, mouthwash, lipid, envelope Disclosure: Venture Life Group plc provided information on mouthwash formulations employed in the study, but had no role in funding, planning, execution, analysis or writing of this study. A separate study funded to Cardiff University by Venture Life Group is assessing in vivo efficacy of CPC in patients with COVID19. The investigators declare no direct conflicts exist. 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.11.13.381079; this version posted November 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. -

Personalized Medicine for Reconstruction of Critical-Size Bone
www.nature.com/npjregenmed ARTICLE OPEN Personalized medicine for reconstruction of critical-size bone defects – a translational approach with customizable vascularized bone tissue ✉ Annika Kengelbach-Weigand 1 , Carolina Thielen 1, Tobias Bäuerle2, Rebekka Götzl 1,5, Thomas Gerber3, Carolin Körner4, Justus P. Beier1,5, Raymund E. Horch 1 and Anja M. Boos1,5 Tissue engineering principles allow the generation of functional tissues for biomedical applications. Reconstruction of large-scale bone defects with tissue-engineered bone has still not entered the clinical routine. In the present study, a bone substitute in combination with mesenchymal stem cells (MSC) and endothelial progenitor cells (EPC) with or without growth factors BMP-2 and VEGF-A was prevascularized by an arteriovenous (AV) loop and transplanted into a critical-size tibia defect in the sheep model. With 3D imaging and immunohistochemistry, we could show that this approach is a feasible and simple alternative to the current clinical therapeutic option. This study serves as proof of concept for using large-scale transplantable, vascularized, and customizable bone, generated in a living organism for the reconstruction of load-bearing bone defects, individually tailored to the patient’s needs. With this approach in personalized medicine for the reconstruction of critical-size bone defects, regeneration of parts of the human body will become possible in the near future. npj Regenerative Medicine (2021) 6:49 ; https://doi.org/10.1038/s41536-021-00158-8 1234567890():,; INTRODUCTION vascular networks consisting of endothelial cells can be created Therapeutic options for bone defects that cannot heal sponta- directly within tissue replacement materials7. Vascularization may neously, the so-called critical-size bone defects, are still limited be further supported by the addition of endothelial cells and and often associated with a great social burden. -
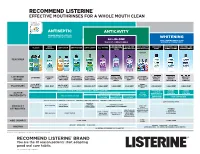
Listerine Floss Product Chart EN V9black
RECOMMEND LISTERINE® EFFECTIVE MOUTHRINSES FOR A WHOLE MOUTH CLEAN® 3 ANTISEPTIC ANTICAVITY 2X MORE HEALTHY SITES VS. JUST BRUSHING & FLOSSING WHITENING ALL-IN-ONE ONLY LEADING BRAND THAT THE MOST COMPLETE RINSE WHITENS & STRENGTHENS ALL-IN-ONE ZERO ALL-IN-ONE ANTI-CAVITY PEROXIDE WHITENS AND WHITENS AND CLASSIC ANTI-STAIN ANTI-TARTAR ANTI-CAVITY ALL-IN-ONE FOR SENSITIVE ALCOHOL TEETH ZERO ALCOHOL ZERO ALCOHOL FREE STRENGTHENS STRENGTHENS METERED DOSING FOR KIDS FEATURES ® LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE TOTAL CARE HEALTHY HEALTHY HEALTHY ULTRACLEAN ULTRACLEAN ULTRACLEAN TOTAL CARE SMART RINSE ZERO TOTAL CARE FOR SENSITIVE WHITE™ WHITE™ WHITE™ BRAND ANTI-STAIN ANTI-TARTAR ANTI-CAVITY TEETH ZERO FOR KIDS GENTLE RESTORING VIBRANT COOL MINT BERRY FRESHBURST ARCTIC MINT BUBBLE GUM FLAVOURS MILD MINT COOL MINT FRESHBURST CLEAN MINT CLEAN MINT MILD MINT CLEAN MINT CLEAN MINT CLEAN MINT ORIGINAL COOL CITRUS MINT EUCALYPTOL 0.091% W/V MENTHOL 0.042% W/V THYMOL 0.063% W/V SODIUM FLUORIDE SODIUM SODIUM 0.02% W/W FLUORIDE FLUORIDE ACTIVE SODIUM FLUORIDE 0.022% W/V SODIUM TETRAPOTASSIUM 0.022% W/V 0.02% W/W FLUORIDE PYROPHOSPHATE INGREDIENTS ZINC CHLORIDE 0.09% W/V ZINC CHLORIDE POTASSIUM ZINC CHLORIDE 0.022% W/V PENTASODIUM HYDROGEN PEROXIDE 0.09% W/V NITRATE 2.4% W/V 0.09% W/V TRIPHOSPHATE KILLS UP TO 99.9% OF GERMS IN YOUR MOUTH PREVENTS & REDUCES GINGIVITIS PREVENTS & REDUCES PLAQUE SAFELY WHITENS TEETH† PREVENTS CAVITIES MILD FLAVOUR HELPS KEEP PREVENTS CAVITIES PRODUCT TEETH WHITE STRENGTHENS STRENGTHENS TOOTH ENAMEL* 2X BETTER TOOTH ENAMEL CAVITY ATTRIBUTES PROTECTION VS. -

July 21, 2021
1 2nd Quarter 2021 Earnings Call July 21, 2021 Cautionary Note on Forward-looking Statements This presentation contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things: future operating and financial performance, product development, market position and business strategy. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to: risks related to the impact of the COVID-19 global pandemic, such as the scope and duration of the outbreak, government actions and restrictive measures implemented in response, material delays and cancellations of medical procedures, supply chain disruptions and other impacts to the business, or on the Company’s ability to execute business continuity plans, as a result of the COVID-19 pandemic; economic factors, such as interest rate and currency exchange rate fluctuations; competition, including technological advances, new products and patents attained by competitors; challenges inherent in new product research and development, including uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new and existing products; challenges to patents; the impact -

HEALTH PROFESSIONAL CONSULTANT to a PHARMACEUTICAL COMPANY V JOHNSON & JOHNSON Nicorette Advertisement
CASE AUTH/2930/1/17 HEALTH PROFESSIONAL CONSULTANT TO A PHARMACEUTICAL COMPANY v JOHNSON & JOHNSON Nicorette advertisement A complaint was received in a private capacity that the implication was that the statement in from a health professional who stated that he/ question related to a feature of Nicorette, that the she worked as a consultant to a pharmaceutical product itself had incredible features and/or that company. health professionals would be doing something incredible by prescribing it. The implication was The complaint concerned an online advertisement misleading and exaggerated and breaches of the for Nicorette (nicotine) issued by Johnson & Code ruled. Johnson published in Pulse. The complainant stated at the time of submitting The complainant provided a screenshot of a the complaint that he/she was a health professional banner advertisement. It included ‘Nicorette. Do who worked as a consultant to Novartis. It had something incredible’. The complainant did not previously been decided, following consideration believe that the word ‘incredible’ was suitable. This by the then Code of Practice Committee and the information did not appear to be balanced and was ABPI Board of Management, that private complaints exaggerated. The claim was taken directly from from pharmaceutical company employees had material aimed at the general public and it appeared to be accepted. To avoid this becoming a means that Johnson & Johnson had not undertaken a of circumventing the normal procedures for sufficiently robust review when translating to intercompany complaints, the employing company promotion aimed at health professionals. would be named in the report. The complainant would be advised that this would happen and be The detailed response from Johnson & Johnson is given an opportunity to withdraw the complaint. -
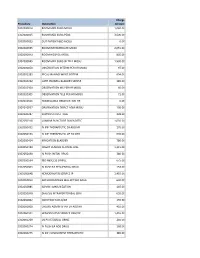
Procedure Description Charge Amount 0302000014 ROOM MED
Charge Procedure Description Amount 0302000014 ROOM MED SURG MOSU 1,040.00 0302000015 ROOM MED SURG PEDS 3,500.00 0302000033 OUT PATIENT BED MOSU 0.00 0302000035 ROOM INTERMEDIATE MOSU 2,055.00 0302000043 ROOM HOSPICE MOSU 805.00 0302000045 ROOM MED SURG W TELE MOSU 1,530.00 0302000050 OBSERVATION INTERM PER HR MOSU 97.00 0302002283 FECAL MANAGEMENT SYSTEM 694.00 0302010232 CATH INDWELL BLADDER SIMPLE 185.00 0302010304 OBSERVATION MS PER HR MOSU 60.00 0302010305 OBSERVATION TELE PER HR MOSU 71.00 0302010556 NONBILLABLE OBSERVATION HR 0.00 0302010557 OBSERVATION DIRECT ADM MOSU 130.00 0302020287 SUPPLIES CHEST TUBE 228.00 0302050318 LUMBAR PUNCTURE DIAGNOSTIC 1,035.00 0302050451 IV INF THERAPEUTIC EA ADD HR 170.00 0302050453 IV INF THERAPEUTIC UP TO 1HR 635.00 0302050454 IRRIGATION BLADDER 780.00 0302050480 INSERT VENOUS CENTRAL LINE 1,315.00 0302050490 IV PUSH INITIAL DRUG 380.00 0302050534 I&D ABSCESS SIMPLE 675.00 0302050603 IV PUSH EA SEQUENTIAL DRUG 153.00 0302050648 HEMODIALYSIS SERVICE IP 1,455.00 0302050813 ARTHROCENTESIS MAJ JNT WO IMAG 630.00 0302050885 ADMIN IMMUNIZATION 145.00 0302050948 DIALYSIS INTRAPERITONEAL SERV 655.00 0302060002 INJECTION SUB-Q/IM 155.00 0302060008 CHEMO ADMIN IV INF EA ADD HR 410.00 0302060101 HEMODIALYSIS SERVICE OBS/OP 1,455.00 0302060269 US PV RESIDUAL URINE 240.00 0302060274 IV PUSH EA ADD DRUG 168.00 0302060275 IV INF CONCURRENT THERAPEUTIC 385.00 0302060276 IV INF SEQUENTIAL THER UP TO 1 191.00 0302060293 INSERT STRAIGHT CATH THERAPEUT 185.00 0302060372 CHEMO ADMIN IV INF SEQ 1 HR 525.00 0302060373 -

Nicorette Invisipatch 25 Mg/16 H Transdermal Patch
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Nicorette invisipatch 25 mg/16 h transdermal patch Nicorette invisipatch 15 mg/16 h transdermal patch Nicorette invisipatch 10 mg/16 h transdermal patch 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each transdermal patch contains nicotine 1.75 mg/cm2. Nicorette invisipatch 25 mg/16 h, of 22.5 cm2 size contains nicotine 39.37 mg and releases nicotine 25 mg /16 hours Nicorette invisipatch 15 mg/16 h, of 13.5 cm2 size contains nicotine 23.62 mg and releases nicotine 15 mg /16 hours Nicorette invisipatch 10 mg/16 h, of 9.0 cm2 size contains nicotine 15.75 mg and releases nicotine 10 mg /16 hours For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Transdermal patch Beige, semi-transparent, rectangular patch with rounded edges and light-brown “Nicorette” printing, is placed on an easily removable layer coated with aluminium and silicon and is formed by nicotine layer and adhesive acrylate layer. 4. CLINICAL PARTICULARS 4.1. Therapeutic indication Nicorette invisipatch is to be used for the treatment of tobacco dependence in adults by relief of nicotine withdrawal symptoms, including cravings, during a quit attempt. Permanent cessation of tobacco use is the eventual objective. Nicorette invisipatch is indicated in adults. Nicorette invisipatch should preferably be used in conjunction with a behavioral support program. 4.2. Posology and method of administration Posology Subjects should stop smoking completely during the course of treatment with Nicorette invisipatch. Administration of nicotine should be stopped immediately if any symptoms of overdose listed in Section 4.9 occur. -

Over-The-Counter Mail Order Program 1-866-768-8490 As a Superior
Over-the-Counter Mail Order Program 1-866-768-8490 As a Superior value-added service, STAR+PLUS and STAR Health members can get $30 in items every 3 months (90 days). STAR members can get $25 in items every 3 months (90 days). No prescription is needed. To order, please call 1-866-768-8490. Have your Superior ID card ready when you call. Your order will be mailed to your home in 5-10 days. Please use these items only as directed. If you have questions about safe use of any of these items, talk to your doctor. Item Description Compare to: Price Item Description Compare to: Price Analgesics Eye Care 1 Ibuprofen 200mg tab Motrin IB $6 31 Tetrahydrozoline drops Visine $4 2 Naproxen sod 220mg tab Aleve $9 61 Lubricating eye drops Refresh Tears $7 3 Aspirin 325mg tab Bayer Aspirin $5 First Aid Creams/Ointments 4 Aspirin ec 325 mg tab Ecotrin $6 32 Calamine lotion Calamine Lotion $4 5 Aspirin ec 81 mg Halfprin $5 33 Hydrocortisone !5 cream Cort-Aid $4 6 Acetaminophen 500mg tab Tylenol Extra Str $6 34 Triple antibiotic ointment Neosporin $5 7 Mentholated ointment Ben Gay $6 60 Medicated lip balm Carmex $3 Antacids First Aid Supplies 8 Simethicone 80mg tab Mylanta Anti-Gas $6 35 Athletic bandage Ace Bandage $7 9 Calc carb 500mg chewable TUMS $6 36 Adhesive tape First-Aid Tape $3 10 Famotidine 10mg tab Pepcid AC $9 37 Band-aids Band-Aids $4 Antidiarrheals 38 Carbamide peroxide Debrox Drops $4 11 Loperamide 2mg cap Imodium $5 39 Gauze pads Gauze Pads $3 12 Bismuth mixture Pepto-Bismol $5 40 Cotton swab Q-Tips $4 Antifungals 41 Oral thermometer Thermometer