Lifescan Ethicon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Learner Notification International Society for Heart & Lung
Learner Notification International Society for Heart & Lung Transplantation (ISHLT) 41st Annual Meeting & Scientific Sessions Virtual Experience April 24 – 28, 2021 Live Virtual Acknowledgement of Financial Commercial Support Abbott Medtronic United Therapeutics Acknowledgement of In-Kind Commercial Support No in-kind commercial support was received for this educational activity. Satisfactory Completion Learners must complete an evaluation form to receive a certificate of completion. Your chosen sessions must be attended in their entirety as partial credit of individual sessions is not available. If you are seeking continuing education credit for a specialty not listed below, it is your responsibility to contact your licensing/certification board to determine course eligibility for your licensing/certification requirement. Physicians (ACCME) The International Society for Heart and Lung Transplantation (ISHLT) is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. Credit Designation Statement - ISHLT designates this live virtual activity for a maximum of 32.00 AMA PRA Category 1 CreditsTM. Physicians should claim only the credit commensurate with the extent of their participation in the activity. Accreditation Statement In support of improving patient care, this activity has been planned and implemented by Amedco LLC and ISHLT. Amedco LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. Nurses (ANCC) - Credit Designation Statement - Amedco LLC designates this live virtual activity for a maximum of 32.00 ANCC contact hours. Pharmacists (ACPE) - Credit Designation Statement - Amedco LLC designates this live virtual activity for a maximum of 32.00 knowledge-based CPE contact hours. -

Mouth Rinses
Mouth Rinses We may recommend a mouth rinse to you for one or more of the following reasons: Periodontal Disease, Gingivitis, Halitosis (bad breath), sensitive teeth, decay or increased risk of decay, dry mouth, minor mouth sore or irritation. Red= RX only Blue= Available through your dentist only Green= Over the counter *Common side effects found with many mouth rinses include the possibility of staining. We still encourage you to use the rinse that treats your problem because none of the stains these rinses cause are permanent. With good oral hygiene on your part, using your bleaching trays and bleach as needed, you can keep the staining to a minimum. If there is any staining that you can’t prevent, it can always be easily removed by your hygienist at your next cleaning. If you have periodontal disease or Gingivitis, we may recommend an antimicrobial/antiseptic type rinse. These rinses kill germs, reduce plaque, redness, swelling and bleeding when used in conjunction with proper brushing and flossing. Some common examples of antiseptic rinses are: Peridex or Perioguard oral rinses are available by prescription only. Active ingredient is chlorhexidine gluconate .12%. These also contain alcohol 11.6%. Can cause staining of the teeth*, taste alteration and dry mouth. Periomed Oral rinse is available through your dentist only. Active ingredient is stannous fluoride .63%. It is alcohol free. Periomed provides antimicrobial activity for up to 8 hours. Also promotes enamel remineralization, and helps reduce sensitivity. Can cause staining* and dry mouth. Listerine Antiseptic: Active ingredient is alcohol 26.9% in the original gold Listerine, and 21.6% in the other flavors. -

2019 Health for Humanity Report
2019 Health for Humanity Report Progress in Sustainability Report Summary Contents Message from Our Chairman and CEO 3 Sustainability Approach 4 2019 Year in Brief 5 Better Health for All: Tackling the World’s 6 Toughest Health Challenges Better Health for All: Access, Community 7 Health & Innovation Responsible Business Practices 8 Environmental Health 9 UNICEF, the Government of Vietnam and Johnson & Johnson are partnering on a national program to train more than 500 ethnic minority midwives in remote regions to provide effective maternal and child health interventions including early essential newborn care in village clinics and homes. Photo by Paul Bettings Front cover Volunteers, frontline health workers and government officials at the launch of the Umurinzi vaccination program in Rwanda. In October 2019, Johnson & Johnson committed to donating up to 700,000 regimens of Janssen’s investigational Ebola vaccine to support the Ebola outbreak response in Rwanda and the Democratic Republic of the Congo. Photos by Rwanda Ministry of Health 2019 Health2017 for Health Humanity for HumanityReport Summary Report 33 Message from Our Chairman and CEO Dear Johnson & Johnson Stakeholders, We know this mission will always be unfinished, and affirmed unequivocally that there is a fundamental that we will occasionally fall short. But that only serves connection between serving all stakeholders and 2019 was a year of profound change and great contrasts as motivation to move faster than we’ve ever moved generating sustainable, long-term value. around the globe. before in making bigger strides toward some of our most ambitious goals. And as we’ve detailed in this Report, we The demands for global healthcare and responsible We saw unprecedented innovation and encouraging have plenty of positive momentum worth recognizing. -

2015 Annual Report
ANNUAL REPORT 2015 MARCH 2016 TO OUR SHAREHOLDERS ALEX GORSKY Chairman, Board of Directors and Chief Executive Officer This year at Johnson & Johnson, we are proud this aligned with our values. Our Board of WRITTEN OVER to celebrate 130 years of helping people Directors engages in a formal review of 70 YEARS AGO, everywhere live longer, healthier and happier our strategic plans, and provides regular OUR CREDO lives. As I reflect on our heritage and consider guidance to ensure our strategy will continue UNITES & our future, I am optimistic and confident in the creating better outcomes for the patients INSPIRES THE long-term potential for our business. and customers we serve, while also creating EMPLOYEES long-term value for our shareholders. OF JOHNSON We manage our business using a strategic & JOHNSON. framework that begins with Our Credo. Written OUR STRATEGIES ARE BASED ON over 70 years ago, it unites and inspires the OUR BROAD AND DEEP KNOWLEDGE employees of Johnson & Johnson. It reminds OF THE HEALTH CARE LANDSCAPE us that our first responsibility is to the patients, IN WHICH WE OPERATE. customers and health care professionals who For 130 years, our company has been use our products, and it compels us to deliver driving breakthrough innovation in health on our responsibilities to our employees, care – from revolutionizing wound care in communities and shareholders. the 1880s to developing cures, vaccines and treatments for some of today’s most Our strategic framework positions us well pressing diseases in the world. We are acutely to continue our leadership in the markets in aware of the need to evaluate our business which we compete through a set of strategic against the changing health care environment principles: we are broadly based in human and to challenge ourselves based on the health care, our focus is on managing for the results we deliver. -

1 Brief Report: the Virucidal Efficacy of Oral Rinse Components Against SARS-Cov-2 in Vitro Evelina Statkute1†, Anzelika Rubin
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.13.381079; this version posted November 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Brief Report: The Virucidal Efficacy of Oral Rinse Components Against SARS-CoV-2 In Vitro Evelina Statkute1†, Anzelika Rubina1†, Valerie B O’Donnell1, David W. Thomas2† Richard J. Stanton1† 1Systems Immunity University Research Institute, Division of Infection & Immunity, School of Medicine, Heath Park, Cardiff, CF14 4XN 2Advanced Therapies Group, School of Dentistry, Cardiff University, Heath Park, Cardiff CF14 4XY, UK †These authors contributed equally * Correspondence: [email protected], [email protected] Running title: Virucidal Activity of Mouthwashes Keywords: SARS-CoV2, mouthwash, lipid, envelope Disclosure: Venture Life Group plc provided information on mouthwash formulations employed in the study, but had no role in funding, planning, execution, analysis or writing of this study. A separate study funded to Cardiff University by Venture Life Group is assessing in vivo efficacy of CPC in patients with COVID19. The investigators declare no direct conflicts exist. 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.11.13.381079; this version posted November 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. -
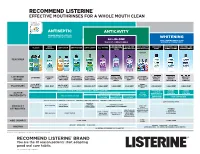
Listerine Floss Product Chart EN V9black
RECOMMEND LISTERINE® EFFECTIVE MOUTHRINSES FOR A WHOLE MOUTH CLEAN® 3 ANTISEPTIC ANTICAVITY 2X MORE HEALTHY SITES VS. JUST BRUSHING & FLOSSING WHITENING ALL-IN-ONE ONLY LEADING BRAND THAT THE MOST COMPLETE RINSE WHITENS & STRENGTHENS ALL-IN-ONE ZERO ALL-IN-ONE ANTI-CAVITY PEROXIDE WHITENS AND WHITENS AND CLASSIC ANTI-STAIN ANTI-TARTAR ANTI-CAVITY ALL-IN-ONE FOR SENSITIVE ALCOHOL TEETH ZERO ALCOHOL ZERO ALCOHOL FREE STRENGTHENS STRENGTHENS METERED DOSING FOR KIDS FEATURES ® LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE TOTAL CARE HEALTHY HEALTHY HEALTHY ULTRACLEAN ULTRACLEAN ULTRACLEAN TOTAL CARE SMART RINSE ZERO TOTAL CARE FOR SENSITIVE WHITE™ WHITE™ WHITE™ BRAND ANTI-STAIN ANTI-TARTAR ANTI-CAVITY TEETH ZERO FOR KIDS GENTLE RESTORING VIBRANT COOL MINT BERRY FRESHBURST ARCTIC MINT BUBBLE GUM FLAVOURS MILD MINT COOL MINT FRESHBURST CLEAN MINT CLEAN MINT MILD MINT CLEAN MINT CLEAN MINT CLEAN MINT ORIGINAL COOL CITRUS MINT EUCALYPTOL 0.091% W/V MENTHOL 0.042% W/V THYMOL 0.063% W/V SODIUM FLUORIDE SODIUM SODIUM 0.02% W/W FLUORIDE FLUORIDE ACTIVE SODIUM FLUORIDE 0.022% W/V SODIUM TETRAPOTASSIUM 0.022% W/V 0.02% W/W FLUORIDE PYROPHOSPHATE INGREDIENTS ZINC CHLORIDE 0.09% W/V ZINC CHLORIDE POTASSIUM ZINC CHLORIDE 0.022% W/V PENTASODIUM HYDROGEN PEROXIDE 0.09% W/V NITRATE 2.4% W/V 0.09% W/V TRIPHOSPHATE KILLS UP TO 99.9% OF GERMS IN YOUR MOUTH PREVENTS & REDUCES GINGIVITIS PREVENTS & REDUCES PLAQUE SAFELY WHITENS TEETH† PREVENTS CAVITIES MILD FLAVOUR HELPS KEEP PREVENTS CAVITIES PRODUCT TEETH WHITE STRENGTHENS STRENGTHENS TOOTH ENAMEL* 2X BETTER TOOTH ENAMEL CAVITY ATTRIBUTES PROTECTION VS. -

July 21, 2021
1 2nd Quarter 2021 Earnings Call July 21, 2021 Cautionary Note on Forward-looking Statements This presentation contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things: future operating and financial performance, product development, market position and business strategy. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to: risks related to the impact of the COVID-19 global pandemic, such as the scope and duration of the outbreak, government actions and restrictive measures implemented in response, material delays and cancellations of medical procedures, supply chain disruptions and other impacts to the business, or on the Company’s ability to execute business continuity plans, as a result of the COVID-19 pandemic; economic factors, such as interest rate and currency exchange rate fluctuations; competition, including technological advances, new products and patents attained by competitors; challenges inherent in new product research and development, including uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new and existing products; challenges to patents; the impact -
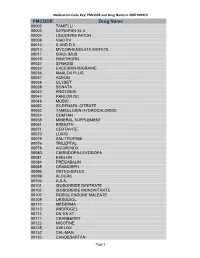
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS Cdc-Pdf
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00002 TAMIFLU 00003 DITROPAN XL II 00004 LIDODERM PATCH 00008 VIACTIV 00010 A AND D II 00013 MYCOPHENOLATE MOFETIL 00017 SIROLIMUS 00019 HAWTHORN 00027 SYNAGIS 00032 EXCEDRIN MIGRAINE 00036 MAALOX PLUS 00037 ACEON 00038 GLYSET 00039 SONATA 00042 PROTONIX 00044 PANLOR DC 00048 MOBIC 00052 SILDENAFIL CITRATE 00053 TAMSULOSIN HYDROCHLORIDE 00054 COMTAN 00058 MINERAL SUPPLEMENT 00061 BISMUTH 00071 CERTAVITE 00073 LUXIQ 00075 SAL-TROPINE 00076 TRILEPTAL 00078 AGGRENOX 00080 CARBIDOPA-LEVODOPA 00081 EXELON 00084 PREGABALIN 00085 ORAMORPH 00096 OSTEO-BIFLEX 00099 ALOCRIL 00100 A.S.A. 00101 ISOSORBIDE DINITRATE 00102 ISOSORBIDE MONONITRATE 00107 ROSIGLITAZONE MALEATE 00109 URSODIOL 00112 MEDERMA 00113 ANDROGEL 00114 DILTIA XT 00117 CRANBERRY 00123 NICOTINE 00125 AVELOX 00132 CAL-MAG 00133 CANDESARTAN Page 1 Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00148 PROLIXIN D 00149 D51/2 NS 00150 NICODERM CQ PATCH 00151 TUSSIN 00152 CEREZYME 00154 CHILDREN'S IBUPROFEN 00156 PROPOXACET-N 00159 KALETRA 00161 BISOPROLOL 00167 NOVOLIN N 00169 KETOROLAC TROMETHAMINE 00172 OPHTHALMIC OINTMENT 00173 ELA-MAX 00176 PREDNISOLONE ACETATE 00179 COLLOID SILVER 00184 KEPPRA 00187 OPHTHALMIC DROPS 00190 ABDEC 00191 HAPONAL 00192 SPECTRAVITE 00198 ENOXAPARIN SODIUM 00206 ACTONEL 00208 CELECOXIB 00209 GLUCOVANCE 00211 LEVALL 5.0 00213 PANTOPRAZOLE SODIUM 00217 TEMODAR 00218 CARBAMIDE PEROXIDE 00221 CHINESE HERBAL MEDS 00224 MILK AND MOLASSES ENEMA 00238 ZOLMITRIPTAN 00239 -

HEALTH PROFESSIONAL CONSULTANT to a PHARMACEUTICAL COMPANY V JOHNSON & JOHNSON Nicorette Advertisement
CASE AUTH/2930/1/17 HEALTH PROFESSIONAL CONSULTANT TO A PHARMACEUTICAL COMPANY v JOHNSON & JOHNSON Nicorette advertisement A complaint was received in a private capacity that the implication was that the statement in from a health professional who stated that he/ question related to a feature of Nicorette, that the she worked as a consultant to a pharmaceutical product itself had incredible features and/or that company. health professionals would be doing something incredible by prescribing it. The implication was The complaint concerned an online advertisement misleading and exaggerated and breaches of the for Nicorette (nicotine) issued by Johnson & Code ruled. Johnson published in Pulse. The complainant stated at the time of submitting The complainant provided a screenshot of a the complaint that he/she was a health professional banner advertisement. It included ‘Nicorette. Do who worked as a consultant to Novartis. It had something incredible’. The complainant did not previously been decided, following consideration believe that the word ‘incredible’ was suitable. This by the then Code of Practice Committee and the information did not appear to be balanced and was ABPI Board of Management, that private complaints exaggerated. The claim was taken directly from from pharmaceutical company employees had material aimed at the general public and it appeared to be accepted. To avoid this becoming a means that Johnson & Johnson had not undertaken a of circumventing the normal procedures for sufficiently robust review when translating to intercompany complaints, the employing company promotion aimed at health professionals. would be named in the report. The complainant would be advised that this would happen and be The detailed response from Johnson & Johnson is given an opportunity to withdraw the complaint. -

Frequently Asked Questions
Frequently Asked Questions Why are these companies included on the "Do Test" list? The following companies manufacture products that are tested on animals at some stage of development. Those marked with a Ƈ are currently observing a moratorium on (i.e., current suspension of) animal testing. Please encourage them to announce a permanent ban. Listed in parentheses are examples of products manufactured by either the company listed or, if applicable, its parent company. For a complete listing of products manufactured by a company on this list, please visit the company's website or contact the company directly for more information. Companies on this list may manufacture individual lines of products that have not been tested on animals. They have not, however, eliminated tests on animals for their entire line of cosmetics and household products. What if a company isn't on either of PETA's lists? Some companies have refused to respond to specific questions about their testing practices. It appears likely that these companies do test on animals at some stage of product development, and their refusal to clarify their testing policies appears to be an attempt to mislead consumers. Other companies may be new. If you find a company not included on our lists, please share the company's contact information with PETA so that we can contact the company directly. Legend Ƈ The company is currently observing a moratorium on animal testing. 3M 3M Corporate 1-888-364-3577 www.solutions.3m.com Headquarters 3M Center St. Paul, Minnesota 55144-1000 Acuvue (Johnson & Johnson) Customer (800) 843-2020 www.acuvue.com/ Relations, D-QA 7500 Centurion Parkway Jacksonville, Florida 32256 Aim (Church & Dwight) P.O. -

Nicorette Invisipatch 25 Mg/16 H Transdermal Patch
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Nicorette invisipatch 25 mg/16 h transdermal patch Nicorette invisipatch 15 mg/16 h transdermal patch Nicorette invisipatch 10 mg/16 h transdermal patch 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each transdermal patch contains nicotine 1.75 mg/cm2. Nicorette invisipatch 25 mg/16 h, of 22.5 cm2 size contains nicotine 39.37 mg and releases nicotine 25 mg /16 hours Nicorette invisipatch 15 mg/16 h, of 13.5 cm2 size contains nicotine 23.62 mg and releases nicotine 15 mg /16 hours Nicorette invisipatch 10 mg/16 h, of 9.0 cm2 size contains nicotine 15.75 mg and releases nicotine 10 mg /16 hours For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Transdermal patch Beige, semi-transparent, rectangular patch with rounded edges and light-brown “Nicorette” printing, is placed on an easily removable layer coated with aluminium and silicon and is formed by nicotine layer and adhesive acrylate layer. 4. CLINICAL PARTICULARS 4.1. Therapeutic indication Nicorette invisipatch is to be used for the treatment of tobacco dependence in adults by relief of nicotine withdrawal symptoms, including cravings, during a quit attempt. Permanent cessation of tobacco use is the eventual objective. Nicorette invisipatch is indicated in adults. Nicorette invisipatch should preferably be used in conjunction with a behavioral support program. 4.2. Posology and method of administration Posology Subjects should stop smoking completely during the course of treatment with Nicorette invisipatch. Administration of nicotine should be stopped immediately if any symptoms of overdose listed in Section 4.9 occur. -

Over-The-Counter Mail Order Program 1-866-768-8490 As a Superior
Over-the-Counter Mail Order Program 1-866-768-8490 As a Superior value-added service, STAR+PLUS and STAR Health members can get $30 in items every 3 months (90 days). STAR members can get $25 in items every 3 months (90 days). No prescription is needed. To order, please call 1-866-768-8490. Have your Superior ID card ready when you call. Your order will be mailed to your home in 5-10 days. Please use these items only as directed. If you have questions about safe use of any of these items, talk to your doctor. Item Description Compare to: Price Item Description Compare to: Price Analgesics Eye Care 1 Ibuprofen 200mg tab Motrin IB $6 31 Tetrahydrozoline drops Visine $4 2 Naproxen sod 220mg tab Aleve $9 61 Lubricating eye drops Refresh Tears $7 3 Aspirin 325mg tab Bayer Aspirin $5 First Aid Creams/Ointments 4 Aspirin ec 325 mg tab Ecotrin $6 32 Calamine lotion Calamine Lotion $4 5 Aspirin ec 81 mg Halfprin $5 33 Hydrocortisone !5 cream Cort-Aid $4 6 Acetaminophen 500mg tab Tylenol Extra Str $6 34 Triple antibiotic ointment Neosporin $5 7 Mentholated ointment Ben Gay $6 60 Medicated lip balm Carmex $3 Antacids First Aid Supplies 8 Simethicone 80mg tab Mylanta Anti-Gas $6 35 Athletic bandage Ace Bandage $7 9 Calc carb 500mg chewable TUMS $6 36 Adhesive tape First-Aid Tape $3 10 Famotidine 10mg tab Pepcid AC $9 37 Band-aids Band-Aids $4 Antidiarrheals 38 Carbamide peroxide Debrox Drops $4 11 Loperamide 2mg cap Imodium $5 39 Gauze pads Gauze Pads $3 12 Bismuth mixture Pepto-Bismol $5 40 Cotton swab Q-Tips $4 Antifungals 41 Oral thermometer Thermometer