2019 SMDP Biotech Training Agenda & Scholar Bios
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mouth Rinses
Mouth Rinses We may recommend a mouth rinse to you for one or more of the following reasons: Periodontal Disease, Gingivitis, Halitosis (bad breath), sensitive teeth, decay or increased risk of decay, dry mouth, minor mouth sore or irritation. Red= RX only Blue= Available through your dentist only Green= Over the counter *Common side effects found with many mouth rinses include the possibility of staining. We still encourage you to use the rinse that treats your problem because none of the stains these rinses cause are permanent. With good oral hygiene on your part, using your bleaching trays and bleach as needed, you can keep the staining to a minimum. If there is any staining that you can’t prevent, it can always be easily removed by your hygienist at your next cleaning. If you have periodontal disease or Gingivitis, we may recommend an antimicrobial/antiseptic type rinse. These rinses kill germs, reduce plaque, redness, swelling and bleeding when used in conjunction with proper brushing and flossing. Some common examples of antiseptic rinses are: Peridex or Perioguard oral rinses are available by prescription only. Active ingredient is chlorhexidine gluconate .12%. These also contain alcohol 11.6%. Can cause staining of the teeth*, taste alteration and dry mouth. Periomed Oral rinse is available through your dentist only. Active ingredient is stannous fluoride .63%. It is alcohol free. Periomed provides antimicrobial activity for up to 8 hours. Also promotes enamel remineralization, and helps reduce sensitivity. Can cause staining* and dry mouth. Listerine Antiseptic: Active ingredient is alcohol 26.9% in the original gold Listerine, and 21.6% in the other flavors. -

Women in Business Awards Luncheon at the Hotel Irvine, Where Aston Martin Americas President Laura Schwab Delivered the Keynote Address
10.5.20 SR_WIB.qxp_Layout 1 10/2/20 12:14 PM Page 29 WOMEN IN BUSINESS NOMINEES START ON PAGE B-60 INSIDE 2019 WINNERS GO BIG IN IRVINE, LAND NEW PARTNERS, INVESTMENTS PAGE 30 PRESENTED BY DIAMOND SPONSOR PLATINUM SPONSORS GOLD SPONSOR SILVER SPONSORS 10.5.20 SR_WIB.qxp_Layout 1 10/2/20 1:36 PM Page 30 30 ORANGE COUNTY BUSINESS JOURNAL www.ocbj.com OCTOBER 5, 2020 Winning Execs Don’t Rest on Their Laurels $1B Cancer Center Underway; Military Wins; Spanish Drug Investment Orange County’s business community last year celebrated the Business Journal’s 25th annual Women in Business Awards luncheon at the Hotel Irvine, where Aston Martin Americas President Laura Schwab delivered the keynote address. The winners, selected from 200 nominees, have not been resting on their laurels, even in the era of the coronavirus. Here are updates on what the five winners have been doing. —Peter J. Brennan Avatar Partners City of Hope Shortly after Marlo Brooke won the Busi- (AR) quality assurance solution for the U.S. As president of the City of Hope Orange employees down from Duarte. Area univer- ness Journal’s award for co-founding Hunt- Navy for aircraft wiring maintenance for the County, Annette Walker is orchestrating a sities could partner with City of Hope. ington Beach-based Avatar Partners Inc., Naval Air Systems Command’s Boeing V- $1 billion project to build one of the biggest, While the larger campus near the Orange she was accepted into the Forbes Technol- 22 Osprey aircraft. and scientifically advanced, cancer research County Great Park is being built, Walker in ogy Council, an invitation-only community Then the Air Force is using Avatar’s solu- centers in the world. -

1 Brief Report: the Virucidal Efficacy of Oral Rinse Components Against SARS-Cov-2 in Vitro Evelina Statkute1†, Anzelika Rubin
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.13.381079; this version posted November 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Brief Report: The Virucidal Efficacy of Oral Rinse Components Against SARS-CoV-2 In Vitro Evelina Statkute1†, Anzelika Rubina1†, Valerie B O’Donnell1, David W. Thomas2† Richard J. Stanton1† 1Systems Immunity University Research Institute, Division of Infection & Immunity, School of Medicine, Heath Park, Cardiff, CF14 4XN 2Advanced Therapies Group, School of Dentistry, Cardiff University, Heath Park, Cardiff CF14 4XY, UK †These authors contributed equally * Correspondence: [email protected], [email protected] Running title: Virucidal Activity of Mouthwashes Keywords: SARS-CoV2, mouthwash, lipid, envelope Disclosure: Venture Life Group plc provided information on mouthwash formulations employed in the study, but had no role in funding, planning, execution, analysis or writing of this study. A separate study funded to Cardiff University by Venture Life Group is assessing in vivo efficacy of CPC in patients with COVID19. The investigators declare no direct conflicts exist. 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.11.13.381079; this version posted November 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. -
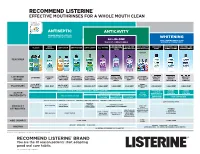
Listerine Floss Product Chart EN V9black
RECOMMEND LISTERINE® EFFECTIVE MOUTHRINSES FOR A WHOLE MOUTH CLEAN® 3 ANTISEPTIC ANTICAVITY 2X MORE HEALTHY SITES VS. JUST BRUSHING & FLOSSING WHITENING ALL-IN-ONE ONLY LEADING BRAND THAT THE MOST COMPLETE RINSE WHITENS & STRENGTHENS ALL-IN-ONE ZERO ALL-IN-ONE ANTI-CAVITY PEROXIDE WHITENS AND WHITENS AND CLASSIC ANTI-STAIN ANTI-TARTAR ANTI-CAVITY ALL-IN-ONE FOR SENSITIVE ALCOHOL TEETH ZERO ALCOHOL ZERO ALCOHOL FREE STRENGTHENS STRENGTHENS METERED DOSING FOR KIDS FEATURES ® LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE TOTAL CARE HEALTHY HEALTHY HEALTHY ULTRACLEAN ULTRACLEAN ULTRACLEAN TOTAL CARE SMART RINSE ZERO TOTAL CARE FOR SENSITIVE WHITE™ WHITE™ WHITE™ BRAND ANTI-STAIN ANTI-TARTAR ANTI-CAVITY TEETH ZERO FOR KIDS GENTLE RESTORING VIBRANT COOL MINT BERRY FRESHBURST ARCTIC MINT BUBBLE GUM FLAVOURS MILD MINT COOL MINT FRESHBURST CLEAN MINT CLEAN MINT MILD MINT CLEAN MINT CLEAN MINT CLEAN MINT ORIGINAL COOL CITRUS MINT EUCALYPTOL 0.091% W/V MENTHOL 0.042% W/V THYMOL 0.063% W/V SODIUM FLUORIDE SODIUM SODIUM 0.02% W/W FLUORIDE FLUORIDE ACTIVE SODIUM FLUORIDE 0.022% W/V SODIUM TETRAPOTASSIUM 0.022% W/V 0.02% W/W FLUORIDE PYROPHOSPHATE INGREDIENTS ZINC CHLORIDE 0.09% W/V ZINC CHLORIDE POTASSIUM ZINC CHLORIDE 0.022% W/V PENTASODIUM HYDROGEN PEROXIDE 0.09% W/V NITRATE 2.4% W/V 0.09% W/V TRIPHOSPHATE KILLS UP TO 99.9% OF GERMS IN YOUR MOUTH PREVENTS & REDUCES GINGIVITIS PREVENTS & REDUCES PLAQUE SAFELY WHITENS TEETH† PREVENTS CAVITIES MILD FLAVOUR HELPS KEEP PREVENTS CAVITIES PRODUCT TEETH WHITE STRENGTHENS STRENGTHENS TOOTH ENAMEL* 2X BETTER TOOTH ENAMEL CAVITY ATTRIBUTES PROTECTION VS. -

July 21, 2021
1 2nd Quarter 2021 Earnings Call July 21, 2021 Cautionary Note on Forward-looking Statements This presentation contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things: future operating and financial performance, product development, market position and business strategy. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to: risks related to the impact of the COVID-19 global pandemic, such as the scope and duration of the outbreak, government actions and restrictive measures implemented in response, material delays and cancellations of medical procedures, supply chain disruptions and other impacts to the business, or on the Company’s ability to execute business continuity plans, as a result of the COVID-19 pandemic; economic factors, such as interest rate and currency exchange rate fluctuations; competition, including technological advances, new products and patents attained by competitors; challenges inherent in new product research and development, including uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new and existing products; challenges to patents; the impact -

Johnson & Johnson Reports 2010 Second-Quarter Results
Johnson & Johnson Reports 2010 Second-Quarter Results: Sales of $15.3 Billion Increased 0.6% Versus 2009 Second-Quarter; EPS was $1.23 Excluding Special Items, 2010 Second-Quarter EPS was $1.21, an increase of 5.2%* NEW BRUNSWICK, N.J., July 20, 2010 /PRNewswire via COMTEX News Network/ -- Johnson & Johnson (NYSE: JNJ) today announced sales of $15.3 billion for the second quarter of 2010, an increase of 0.6% as compared to the second quarter of 2009. Operational results increased 0.1% and the positive impact of currency was 0.5%. Domestic sales declined 2.8%, while international sales increased 4.1%, reflecting operational growth of 3.0% and a positive currency impact of 1.1%. Net earnings and diluted earnings per share for the second quarter of 2010 were $3.4 billion and $1.23, respectively. Second- quarter 2010 net earnings included an after-tax gain of $67 million representing the net impact of litigation matters. Excluding this special item, net earnings for the current quarter were $3.4 billion and diluted earnings per share were $1.21, representing increases of 5.4% and 5.2%, respectively, as compared to the same period in 2009.* The Company updated its earnings guidance for full-year 2010 to $4.65 - $4.75 per share, which excludes the impact of special items. The Company's guidance now reflects the impact of the voluntary recalls announced earlier this year of certain over-the-counter medicines and the suspension of manufacturing at the McNeil Consumer Healthcare facility in Fort Washington, Pa., as well as unfavorable changes in foreign currency exchange rates. -
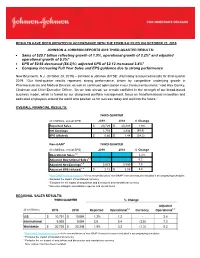
Sales of $20.7 Billion Reflecting Growth of 1.9%, Operational Growth of 3.2
RESULTS HAVE BEEN UPDATED IN ACCORDANCE WITH THE FORM 8-K FILED ON OCTOBER 23, 2019 JOHNSON & JOHNSON REPORTS 2019 THIRD-QUARTER RESULTS: • Sales of $20.7 billion reflecting growth of 1.9%, operational growth of 3.2%* and adjusted operational growth of 5.2%* • EPS of $0.66 decreased (54.2)%; adjusted EPS of $2.12 increased 3.4%* • Company increasing Full Year Sales and EPS guidance due to strong performance New Brunswick, N.J. (October 23, 2019) – Johnson & Johnson (NYSE: JNJ) today announced results for third-quarter 2019. “Our third-quarter results represent strong performance, driven by competitive underlying growth in Pharmaceuticals and Medical Devices, as well as continued optimization in our Consumer business,” said Alex Gorsky, Chairman and Chief Executive Officer. “As we look ahead, we remain confident in the strength of our broad-based business model, which is fueled by our disciplined portfolio management, focus on transformational innovation and dedicated employees around the world who position us for success today and well into the future.” OVERALL FINANCIAL RESULTS: THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Reported Sales $ 20,729 $ 20,348 1.9% Net Earnings 1,753 3,934 (55.4) EPS (diluted) $ 0.66 $ 1.44 (54.2) Non-GAAP* THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Operational Sales1,2 3.2% Adjusted Operational Sales1,3 5.2 Adjusted Net Earnings1,4 5,672 5,590 1.5 Adjusted EPS (diluted)1,4 $ 2.12 $ 2.05 3.4 1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures -

Department of Public Health 105 Cmr 720.000
105 CMR: DEPARTMENT OF PUBLIC HEALTH 105 CMR 720.000: LIST OF INTERCHANGEABLE DRUG PRODUCTS Section 720.001: Purpose 720.002: Citation 720.010: Scope and Application 720.020: Definitions Standards 720:040: Commission Review of Relevant Drug Products 720.050: List of Interchangeable Drug Products 720.060: Drug Products Excluded 720.070: Amendments to the Massachusetts List of Interchangeable Drugs Procedures for Amending List of Interchangeable Drug Products 720.080: Procedures for Amending the Massachusetts List of Interchangeable Drugs 720.081: Petition to Amend List of Interchangeable Drug Products 720.082: Commission Review of Petition 720.083: Notice of Public Comment Period 720.084: Commission Recommendation of Amendments to Department 720.090: Department Adoption of Amendments 720.100: Severability 720.200: Appendix A: List of Interchangeable Drugs 720.001: Purpose The purpose of 105 CMR 720.000 is to establish a drug formulary, or list of interchangeable drug products, for use by physicians, other practitioners, and pharmacists licensed to practice within the commonwealth, so that consumers of prescription drug products may realize cost savings by buying less expensive, safe drug products. 720.002: Citation 105 CMR 720.000 shall be known as the 105 CMR 720.000: Massachusetts List of Interchangeable Drug Products. 720.010: Scope and Application 105 CMR 720.000 establishes the list of interchangeable drug products from which a pharmacist must interchange a reasonably available less expensive drug product than that written, when a prescription written by a practitioner indicates "interchange". 105 CMR 720.000 also establishes criteria and procedures for inclusion of drug products on this list. -

Milestone Guide and Workbook
Tulsa County Family Treatment Court Milestone Guide and Workbook 6th Edition May 2019 Tulsa County Family Treatment Court Table of Contents Milestone 0 Orientation Milestone 0 Checklist 3 Family Treatment Court Contract 4-6 Consent for Re-disclosure of Confidential Information 7 Medical Compliance Form 8 Family Treatment Court Countdown to Sobriety Worksheet 9 Drug Testing Program Requirements 10 Urine Analysis Collection Policy and Procedures 11 Courtroom Rules and Treatment Court Important Points to Remember 12 Client Information Sheet 13 Court Dress Code 14 Prescription Drug Policy 15-16 Doctors, Medicine, and Family Treatment Court Information 17 Approved Over the Counter Medication List 18 Milestone 1 White Belt Milestone 1 Checklist 19-20 Important Contact Information 21 Appointment Scheduler Worksheet 22 Rules for Mentees and Mentors 23-24 What are You Strengths? 25 Taking Care of Me 26 Appendix A 211 Information Packet Appendix B Narcotics Anonymous and Alcoholics Anonymous Meeting Information 2 Tulsa County Family Treatment Court Milestone 0 White Belt Orientation and Introduction Drug Test Color: Gold Completed orientation packet o Contract o Release of Confidential Information o Medical Compliance o Drug Testing Program Requirements o UA Collection o Client Information Sheet Received o Courtroom Rules o Dress Code o Prescription Drug Policy o Doctor, Medicine, and FTC info sheet Received White wrist band Issued FTC Guidebook binder Recognized to court. You starting court date is: Sober date: Your date to start drug testing is: The color line number is 918-596-8737 Drug testing is done at DATL o (2626 S. Sheridan, phone: 918-664-3285) 3 Tulsa County Family Treatment Court Family Treatment Court Contract I, _______________________________, have been accepted as a voluntary participant in the Tulsa County Family Treatment Court Program. -

Exhibitor & Sponsor Program
Exhibitor & Sponsor Program www.AtlanticDermConference.org @AtlanticDermADC The Philadelphia Dermatological Society and the Atlantic Derm Conference are pleased to acknowledge and express our sincere appreciation for our corporate sponsors, exhibitors, and commercial supporters. Disclosure of Commercial Support | This activity is supported by an educational donation provided by Amgen, an educational grant provided by Galderma Laboratories, LP, and an educational grant provided by Valeant Dermatology. SPONSORS DIAMOND PLATINUM GOLD SILVER Pharmaceuticals North America LLC www.AtlanticDermConference.org | Page 1 " !"!%! "%#%! !! !#! !% "!! #!!#%$ %"!"! ! !!!! " !%"!! '!!!&! ! "! ' !!! !!"!% "%# ! ( ! # ' % "& ## #!!#" #"$" Exhibitors Exhibitors 3Gen, Inc. | 31521 Rancho Viejo Road, #104, San Juan Capistrano, CA 92675 | 949.481.6384 | www.dermlite.com Table #15 ADDRESSING 3Gen manufactures the DermLite brand of skin imaging devices. THE TOUGHEST AbbVie | 1 N. Waukegan Road, North Chicago, IL 60064 | 847.937.7390 | abbvie.com | www.psoriasis.com Table #1 & 2 AbbVie is a global, research-based biopharmaceutical company which combines the focus of a leading-edge biotech with DERMATOLOGY the expertise and structure of a long-established pharmaceutical leader. AbbVie is committed to using unique approaches to innovation to develop and market advanced therapies that address some of the world’s most complex, serious diseases. CHALLENGES Actelion Pharmaceuticals US, Inc. | 5000 Shoreline Ct., South San Francisco, -

Tips for Managing Treatment-Related Rash and Dry Skin
RASH Tips for Managing Treatment-Related Rash and Dry Skin Presented by Stewart B. Fleishman, MD Continuum Cancer Centers of New York: Beth Israel & St. Luke’s-Roosevelt Lindy P. Fox, MD University of California San Francisco David H. Garfield, MD University of Colorado Comprehensive Cancer Center Carol S. Viele, RN, MS University of California San Francisco Carolyn Messner, DSW CancerCare Learn about: • Effects of targeted treatments on the skin • Managing rashes and dry skin • Treating nail conditions • Your support team Help and Hope CancerCare is a national nonprofit organization that provides free support services to anyone affected by cancer: people with cancer, caregivers, children, loved ones, and the bereaved. CancerCare programs—including counseling and support groups, education, financial assistance, and practical help—are provided by professional oncology social workers and are completely free of charge. Founded in 1944, CancerCare provided individual help to more than 100,000 people last year and had more than 1 million unique visitors to our websites. For more information, call 1-800-813-HOPE (4673) or visit www.cancercare.org. Contacting CancerCare National Office Administration CancerCare Tel: 212-712-8400 The material presented in this patient booklet is provided for your general 275 Seventh Avenue Fax: 212-712-8495 information only. It is not intended as medical advice and should not be relied New York, NY 10001 Email: [email protected] upon as a substitute for consultations with qualified health professionals who Email: [email protected] Website: www.cancercare.org are aware of your specific situation. We encourage you to take information and Services questions back to your individual health care provider as a way of creating a Tel: 212-712-8080 dialogue and partnership about your cancer and your treatment. -

TITLE Safety and Efficacy of Over-The-Counter Drug Use by the Elderly
" DOCUMENT RESUME ED 245 148 CG 017 518 TITLE Safety and Efficacy of Over-the-Counter Drug Use by the Elderly. Hearing before the Subcommittee on Health and Long-Term Care of the Select Committee on Aging. House of Representatives, Ninety-Eighth Congress, First Session. INSTITUTION Congress of the U.S., Washington, D.C. House Select Committee on Aging. REPORT NO House-Comm-Pub-98-409 PUB DATE 21 Jul 83 NOTE 507p.; Some pages are marginally legible due to small print. PUB TYPE Legal/Legislative/Regulatory Materials (090) EDRS PRICE MF02 Plus Postage. PC Not Available from EDRS. DESCRIPTORS *Consumer Protection; Dietetics; Drug Legislation; *Drug Use; Gerontology; Hearings; *Older Adults; Physical Health; *Safety IDENTIFIERS Congress 98th; Long Term Care; *Nonprescription Drugs; *PPA ABSTRACT This document contains the prepared statements and panel testimony from the Congressional hearing on over-the-counter (OTC) drug use by the elderly. Opening statements are given by Representatives Claude Pepper (chairman), Ralph Regula, Mary Rose Oakar, Michael Bilirakis, Tom Lantos, and Hal Daub. Topics which are covered include the incidence and quantity of drug use by the elderly, health risks, adverse reactions, phenylpropanolamine (PPA), consumer protection, and the Federal Drug Administration's (FDA) role in the OTC drug safety and reguIatiem. Testimony of the first panel on OTC drugs, particularly weight reduction medications containing PPA, is given by representatives of the Health Research Group, the National Broadcasting Company, and consumers. Testimony of the second panel on mail fraud schemes perpetrated against senior citizens is given by consumer advocates representing the United States Postal Service, Criminal Investigations and Consumer Protection Divisions, and the Center for Science in the Public Interest.