Department of Public Health 105 Cmr 720.000
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Women in Business Awards Luncheon at the Hotel Irvine, Where Aston Martin Americas President Laura Schwab Delivered the Keynote Address
10.5.20 SR_WIB.qxp_Layout 1 10/2/20 12:14 PM Page 29 WOMEN IN BUSINESS NOMINEES START ON PAGE B-60 INSIDE 2019 WINNERS GO BIG IN IRVINE, LAND NEW PARTNERS, INVESTMENTS PAGE 30 PRESENTED BY DIAMOND SPONSOR PLATINUM SPONSORS GOLD SPONSOR SILVER SPONSORS 10.5.20 SR_WIB.qxp_Layout 1 10/2/20 1:36 PM Page 30 30 ORANGE COUNTY BUSINESS JOURNAL www.ocbj.com OCTOBER 5, 2020 Winning Execs Don’t Rest on Their Laurels $1B Cancer Center Underway; Military Wins; Spanish Drug Investment Orange County’s business community last year celebrated the Business Journal’s 25th annual Women in Business Awards luncheon at the Hotel Irvine, where Aston Martin Americas President Laura Schwab delivered the keynote address. The winners, selected from 200 nominees, have not been resting on their laurels, even in the era of the coronavirus. Here are updates on what the five winners have been doing. —Peter J. Brennan Avatar Partners City of Hope Shortly after Marlo Brooke won the Busi- (AR) quality assurance solution for the U.S. As president of the City of Hope Orange employees down from Duarte. Area univer- ness Journal’s award for co-founding Hunt- Navy for aircraft wiring maintenance for the County, Annette Walker is orchestrating a sities could partner with City of Hope. ington Beach-based Avatar Partners Inc., Naval Air Systems Command’s Boeing V- $1 billion project to build one of the biggest, While the larger campus near the Orange she was accepted into the Forbes Technol- 22 Osprey aircraft. and scientifically advanced, cancer research County Great Park is being built, Walker in ogy Council, an invitation-only community Then the Air Force is using Avatar’s solu- centers in the world. -

Johnson & Johnson Reports 2010 Second-Quarter Results
Johnson & Johnson Reports 2010 Second-Quarter Results: Sales of $15.3 Billion Increased 0.6% Versus 2009 Second-Quarter; EPS was $1.23 Excluding Special Items, 2010 Second-Quarter EPS was $1.21, an increase of 5.2%* NEW BRUNSWICK, N.J., July 20, 2010 /PRNewswire via COMTEX News Network/ -- Johnson & Johnson (NYSE: JNJ) today announced sales of $15.3 billion for the second quarter of 2010, an increase of 0.6% as compared to the second quarter of 2009. Operational results increased 0.1% and the positive impact of currency was 0.5%. Domestic sales declined 2.8%, while international sales increased 4.1%, reflecting operational growth of 3.0% and a positive currency impact of 1.1%. Net earnings and diluted earnings per share for the second quarter of 2010 were $3.4 billion and $1.23, respectively. Second- quarter 2010 net earnings included an after-tax gain of $67 million representing the net impact of litigation matters. Excluding this special item, net earnings for the current quarter were $3.4 billion and diluted earnings per share were $1.21, representing increases of 5.4% and 5.2%, respectively, as compared to the same period in 2009.* The Company updated its earnings guidance for full-year 2010 to $4.65 - $4.75 per share, which excludes the impact of special items. The Company's guidance now reflects the impact of the voluntary recalls announced earlier this year of certain over-the-counter medicines and the suspension of manufacturing at the McNeil Consumer Healthcare facility in Fort Washington, Pa., as well as unfavorable changes in foreign currency exchange rates. -
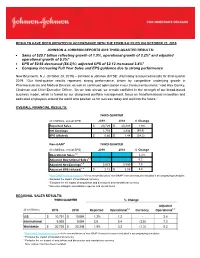
Sales of $20.7 Billion Reflecting Growth of 1.9%, Operational Growth of 3.2
RESULTS HAVE BEEN UPDATED IN ACCORDANCE WITH THE FORM 8-K FILED ON OCTOBER 23, 2019 JOHNSON & JOHNSON REPORTS 2019 THIRD-QUARTER RESULTS: • Sales of $20.7 billion reflecting growth of 1.9%, operational growth of 3.2%* and adjusted operational growth of 5.2%* • EPS of $0.66 decreased (54.2)%; adjusted EPS of $2.12 increased 3.4%* • Company increasing Full Year Sales and EPS guidance due to strong performance New Brunswick, N.J. (October 23, 2019) – Johnson & Johnson (NYSE: JNJ) today announced results for third-quarter 2019. “Our third-quarter results represent strong performance, driven by competitive underlying growth in Pharmaceuticals and Medical Devices, as well as continued optimization in our Consumer business,” said Alex Gorsky, Chairman and Chief Executive Officer. “As we look ahead, we remain confident in the strength of our broad-based business model, which is fueled by our disciplined portfolio management, focus on transformational innovation and dedicated employees around the world who position us for success today and well into the future.” OVERALL FINANCIAL RESULTS: THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Reported Sales $ 20,729 $ 20,348 1.9% Net Earnings 1,753 3,934 (55.4) EPS (diluted) $ 0.66 $ 1.44 (54.2) Non-GAAP* THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Operational Sales1,2 3.2% Adjusted Operational Sales1,3 5.2 Adjusted Net Earnings1,4 5,672 5,590 1.5 Adjusted EPS (diluted)1,4 $ 2.12 $ 2.05 3.4 1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures -

Exhibitor & Sponsor Program
Exhibitor & Sponsor Program www.AtlanticDermConference.org @AtlanticDermADC The Philadelphia Dermatological Society and the Atlantic Derm Conference are pleased to acknowledge and express our sincere appreciation for our corporate sponsors, exhibitors, and commercial supporters. Disclosure of Commercial Support | This activity is supported by an educational donation provided by Amgen, an educational grant provided by Galderma Laboratories, LP, and an educational grant provided by Valeant Dermatology. SPONSORS DIAMOND PLATINUM GOLD SILVER Pharmaceuticals North America LLC www.AtlanticDermConference.org | Page 1 " !"!%! "%#%! !! !#! !% "!! #!!#%$ %"!"! ! !!!! " !%"!! '!!!&! ! "! ' !!! !!"!% "%# ! ( ! # ' % "& ## #!!#" #"$" Exhibitors Exhibitors 3Gen, Inc. | 31521 Rancho Viejo Road, #104, San Juan Capistrano, CA 92675 | 949.481.6384 | www.dermlite.com Table #15 ADDRESSING 3Gen manufactures the DermLite brand of skin imaging devices. THE TOUGHEST AbbVie | 1 N. Waukegan Road, North Chicago, IL 60064 | 847.937.7390 | abbvie.com | www.psoriasis.com Table #1 & 2 AbbVie is a global, research-based biopharmaceutical company which combines the focus of a leading-edge biotech with DERMATOLOGY the expertise and structure of a long-established pharmaceutical leader. AbbVie is committed to using unique approaches to innovation to develop and market advanced therapies that address some of the world’s most complex, serious diseases. CHALLENGES Actelion Pharmaceuticals US, Inc. | 5000 Shoreline Ct., South San Francisco, -

Tips for Managing Treatment-Related Rash and Dry Skin
RASH Tips for Managing Treatment-Related Rash and Dry Skin Presented by Stewart B. Fleishman, MD Continuum Cancer Centers of New York: Beth Israel & St. Luke’s-Roosevelt Lindy P. Fox, MD University of California San Francisco David H. Garfield, MD University of Colorado Comprehensive Cancer Center Carol S. Viele, RN, MS University of California San Francisco Carolyn Messner, DSW CancerCare Learn about: • Effects of targeted treatments on the skin • Managing rashes and dry skin • Treating nail conditions • Your support team Help and Hope CancerCare is a national nonprofit organization that provides free support services to anyone affected by cancer: people with cancer, caregivers, children, loved ones, and the bereaved. CancerCare programs—including counseling and support groups, education, financial assistance, and practical help—are provided by professional oncology social workers and are completely free of charge. Founded in 1944, CancerCare provided individual help to more than 100,000 people last year and had more than 1 million unique visitors to our websites. For more information, call 1-800-813-HOPE (4673) or visit www.cancercare.org. Contacting CancerCare National Office Administration CancerCare Tel: 212-712-8400 The material presented in this patient booklet is provided for your general 275 Seventh Avenue Fax: 212-712-8495 information only. It is not intended as medical advice and should not be relied New York, NY 10001 Email: [email protected] upon as a substitute for consultations with qualified health professionals who Email: [email protected] Website: www.cancercare.org are aware of your specific situation. We encourage you to take information and Services questions back to your individual health care provider as a way of creating a Tel: 212-712-8080 dialogue and partnership about your cancer and your treatment. -

2019 SMDP Biotech Training Agenda & Scholar Bios
2019 SMDP Biotech 1-5 June PHILADELPHIA www.icpdprograms.org 2019 Scientist Mentoring & Diversity Program for biotechnology (SMDP Biotech) www.icpdprograms.org Training Session June 1-6, 2019 in Philadelphia, PA about the program who attends: the one-year career mentoring program pairs ethnically diverse post-baccalaureate students, graduate students and post-doctoral researchers with industry mentors who work at biotechnology and consumer healthcare companies. With their mentors, SMDP Biotech Scholars attend a 5-day training session to learn about career opportunities in industry and receive career coaching. SMDP Scholars and Mentors also attend The BIO International Convention. how to dress, what to bring: business attire with comfortable shoes. Scholars, bring 100 business cards and 10 copies of your resume. Mentors will need business cards too. Informal dinner where to go: SMDP training Scholars and Mentors you’re Host Hotel session day 1 already on the guest list, we Mentors you will arrange the bus leaves the leave J&J Consumer at 5:15pm your own accommodation host hotel at 6:30am Ladder 15, 1528 Sansom Street, Scholars a shared room Scholars you will be Philadelphia, PA 19102 reservation has already been introduced to your made for you at this location: Mentors at the Reading Terminal MarKet Philadelphia 201, 201 N. 17th training session The bus leaves from the SMDP J&J Consumer, Street, Philadelphia, PA 19103 training session at 11:30am 199 Grandview Road, Corner of North 12th Street “Celebration of Mentoring Skillman, NJ 08558 and Arch Street (South Bldg. SK1912) & Diversity” reception ($10 will be provided) (Saturday, June 1st at 6pm) SMDP training The BIO Intl Convention Scholars and Mentors you’re session day 2 registration information will be already on the guest list the bus leaves the provided during the SMDP The Pyramid Club, host hotel at 7am training session. -

Propylene Glycol
PROPYLENE GLYCOL Your patch test result indicates that you have a contact allergy to propylene glycol. This contact allergy may cause your skin to react when it is exposed to this substance although it may take several days for the symptoms to appear. Typical symptoms include redness, swelling, itching, and fluid-filled blisters. Where is propylene glycol found? Propylene glycol is used as a softening agent, preservative, humectants, and solvent in cosmetics, fragrances, topical medications, soaps and cleansers, hair care products, and deodorants. Propylene glycol is also found in oral treatments as well as many foods. It is also added during the manufacture of many industrial fluids, such as solvents, thinners, antifreeze, other de-icing fluids, desiccants, brake fluids, and polyester resins. How can you avoid contact with propylene glycol? Avoid products that list any of the following names in the ingredients: • Propylene glycol • 1,2-Dihydroxypropane • CASRN: 57-55-6 • Methylethyl glycol • 1,2-Propanediol • 2-Hydroxypropanol • Isopropylene glycol What are some products that may contain propylene glycol? Antiperspirants and Deodorants: • Old Spice High Endurance • Meguiars Vinyl/Rubber Cleaner/Condition • Adidas 24 Hour Deodorant Control Antiperspirant & Deodorant • Pennzoil Roadside Fix A Flat Tire Sealant & • Adidas 24 Hour Fragrance Clear Stick • Old Spice High Endurance Deodorant Flat Preventative Deodorant • Old Spice Red Zone Clear Gel • Rain-X De-Icer (Aerosol) • Adidas Action 3 Tech F • Old Spice Red Zone Deodorant Stick • Slime -

Johnson & Johnson Reports 2008 Second-Quarter Results
Johnson & Johnson Reports 2008 Second-Quarter Results: Sales of $16.5 Billion Increased 8.7% Versus a Year Ago; EPS was $1.17 Excluding 2008 Special Items, Second-Quarter EPS of $1.18 Increased 12.4%* NEW BRUNSWICK, N.J., July 15, 2008 /PRNewswire-FirstCall via COMTEX News Network/ -- Johnson & Johnson today announced record sales of $16.5 billion for the second quarter of 2008, an increase of 8.7% as compared to the second quarter of 2007. Operational growth was 3.1% and the positive impact of currency was 5.6%. Domestic sales were up 2.1%, while international sales increased 16.2%, reflecting operational growth of 4.3% and a positive currency impact of 11.9%. Net earnings and diluted earnings per share for the second quarter of 2008 were $3.3 billion and $1.17, respectively. The second quarter of 2008 included an after-tax in-process research and development charge of $40 million associated with the acquisition of Amic, a developer of in vitro diagnostic technologies for use in Point-of-Care and near-patient settings. Excluding this charge, net earnings for the quarter of $3.4 billion and diluted earnings per share of $1.18 represent increases of 9.3% and 12.4 %, respectively, as compared to the same period in 2007.* The Company increased its earnings guidance for full- year 2008 to $4.45 - $4.50 per share, which does not include the impact of any in-process research and development charges or other special items. "Our solid earnings this quarter build on our strong track record of performance," said William C. -

Annual Report
ANNUAL REPORT 2019 MARCH 2020 To Our Shareholders Alex Gorsky Chairman and Chief Executive Officer By just about every measure, Johnson & These are some of the many financial and Johnson’s 133rd year was extraordinary. strategic achievements that were made possible by the commitment of our more than • We delivered strong operational revenue and 132,000 Johnson & Johnson colleagues, who adjusted operational earnings growth* that passionately lead the way in improving the health exceeded the financial performance goals we and well-being of people around the world. set for the Company at the start of 2019. • We again made record investments in research and development (R&D)—more than $11 billion across our Pharmaceutical, Medical Devices Propelled by our people, products, and and Consumer businesses—as we maintained a purpose, we look forward to the future relentless pursuit of innovation to develop vital with great confidence and optimism scientific breakthroughs. as we remain committed to leading • We proudly launched new transformational across the spectrum of healthcare. medicines for untreated and treatment-resistant diseases, while gaining approvals for new uses of many of our medicines already in the market. Through proactive leadership across our enterprise, we navigated a constant surge • We deployed approximately $7 billion, of unique and complex challenges, spanning primarily in transactions that fortify our dynamic global issues, shifting political commitment to digital surgery for a more climates, industry and competitive headwinds, personalized and elevated standard of and an ongoing litigious environment. healthcare, and that enhance our position in consumer skin health. As we have experienced for 133 years, we • And our teams around the world continued can be sure that 2020 will present a new set of working to address pressing public health opportunities and challenges. -

Keep Your Skin Clean and Well Moisturized
Callan-Harris Physical Therapy, P.C. Skin Care: Keep your skin clean and well moisturized. Maintain good hygiene and condition. There is a hydrolipid film of the epidermis – a mixture of water and lipids – that is created from the secretions of the sebaceous glands and the sweat that coats the skin and protects it from exogenous influences. (pH 4.5 and 5.7) (i.e. microbial colonization among other things) Reduces water evaporation and keeps the skin supple. We want to maintain this layer by using a soap free cleanser with a pH of 7 or slightly acidic (appox. pH 5). Below are some products with pH levels to compare. Sometimes in the clinic we use black tea (pH 4.9) AHAVA Soaps 5.5 MD Forte Facial Cleanser II 15%GA 3.8 Alpha Hydrox Foaming Face Wash 6.1-6.5 MD Formulations Sensitive Skin Cleanser 4.4 Alpha Hydrox Optimum Series Moisturizing Cleanser 6.3-6.5 MD Formulations Basic Facial Cleanser 5.5 Beauty Without Cruelty Vitamin C Cleanser 6.0-6.5 MD Formulations Oily/Problem Cleanser 3.8 Burt’s Bees Tomato, Carrot, and Lettuce soaps 10 Neutrogena Deep Clean Facial Cleanser 3.8-4.6 Camay Soap 9.5 Neutrogena Deep Clean Cream Cleanser 2.8-3.8 Camocare Gold Light Foaming Cleanser 6.5-7.0 Neutrogena Deep Clean Cleansing Cloths N/A Cellex-C Betaplex Cleanser 5.0-5.5 Neutrogena Extra Gentle Cleanser 5.6-6.2 Cetaphil cleanser 6.7 Neutrogena Extra Gentle Cleansing Bar 6.0-7.5 Dermalogica Exfoliants: 3.4-3.9 Neutrogena Facial Cleansing Bar Original Formula 8.7-9.2 Dermalogica The Bar 5.5 Neutrogena Fresh Foaming Cleanser 6.2-6.9 Dermalogica Dermal Clay Cleanser 6.2 Liquid Neutrogena 8.7-9.1 Dial Soap (liquid and bar) 9.5 Neutrogena Pore Refining Cleanser 3.7-4.2 Dove Bar, Baby Dove Bar 7 Neutrogena Cleansing Bar for Acne-Prone Skin 8.7-9.2 Dr. -

JNJ Earnings Presentation 4Q2016
1 4th Quarter 2016 Earnings Call Presentation January 24, 2017 2 Joseph J. Wolk Vice President Investor Relations 3 Cautionary Note on Forward-Looking Statements These presentations contain “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, future operating and financial performance, product development, market position and business strategy. The viewer is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to, economic factors, such as interest rate and currency exchange rate fluctuations; competition, including technological advances, new products and patents attained by competitors; challenges inherent in product research and development, including uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new and existing products; challenges to patents; the impact of patent expirations; the ability of the company to successfully execute strategic plans, including business plans, transactions and restructuring plans; market conditions and the possibility that the on-going share repurchase program may be delayed, suspended or discontinued; the impact of business combinations and divestitures; -

Ronald Monk, Et Al. V. Johnson & Johnson, Et Al. 10-CV-04841
James E. Cecchi D. Seamus Kaskela Lindsey H. Taylor David M. Promisloff CARELLA, BYRNE, CECCHI, BARROWAY TOPAZ KESSLER OLSTEIN, BRODY & AGNELLO, P.C. MELTZER & CHECK, LLP 5 Becker Farm Road 280 King of Prussia Road Roseland, New Jersey 07068 Radnor, Pennsylvania 19087 (973) 994-1700 (610) 667-7706 Ramzi Abadou Erik D. Peterson Stacey Kaplan BARROWAY TOPAZ KESSLER MELTZER & CHECK, LLP 580 California Street, Suite 1750 San Francisco, California 94104 (415) 400-3000 Attorneys for Plaintiff UNITED STATES DISTRICT COURT DISTRICT OF NEW JERSEY Civil Action No. RONALD MONK, Individually and on Behalf of All Others Similarly Situated, Plaintiff, vs. COMPLAINT and DEMAND FOR JURY TRIAL JOHNSON & JOHNSON, WILLIAM C. WELDON, DOMINIC J. CARUSO and PETER LUTHER, Defendants. Plaintiff Ronald Monk, individually and on behalf of others similarly situated, by way of Complaint against Johnson & Johnson, William C. Weldon, Dominic J. Caruso, and Peter Luther, say: INTRODUCTION AND OVERVIEW 1. This is a class action for violations of the anti-fraud provisions of the federal securities laws on behalf of all purchasers of Johnson & Johnson (“JNJ” or the “Company”) publicly traded securities between October 14, 2008 and July 21, 2010 (the “Class Period”), who were damaged thereby. 2. JNJ is a healthcare company that sells, among other things, over-the-counter (“OTC”) drugs to consumers. During the Class Period, defendants repeatedly touted the success and revenue of OTC drug sales by its subsidiary McNeil-PPC, Inc. (“McNeil”). At the same time, defendants failed to disclose that a number of manufacturing facilities were failing to maintain current good manufacturing practices (“cGMPs”).