Ronald Monk, Et Al. V. Johnson & Johnson, Et Al. 10-CV-04841
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Women in Business Awards Luncheon at the Hotel Irvine, Where Aston Martin Americas President Laura Schwab Delivered the Keynote Address
10.5.20 SR_WIB.qxp_Layout 1 10/2/20 12:14 PM Page 29 WOMEN IN BUSINESS NOMINEES START ON PAGE B-60 INSIDE 2019 WINNERS GO BIG IN IRVINE, LAND NEW PARTNERS, INVESTMENTS PAGE 30 PRESENTED BY DIAMOND SPONSOR PLATINUM SPONSORS GOLD SPONSOR SILVER SPONSORS 10.5.20 SR_WIB.qxp_Layout 1 10/2/20 1:36 PM Page 30 30 ORANGE COUNTY BUSINESS JOURNAL www.ocbj.com OCTOBER 5, 2020 Winning Execs Don’t Rest on Their Laurels $1B Cancer Center Underway; Military Wins; Spanish Drug Investment Orange County’s business community last year celebrated the Business Journal’s 25th annual Women in Business Awards luncheon at the Hotel Irvine, where Aston Martin Americas President Laura Schwab delivered the keynote address. The winners, selected from 200 nominees, have not been resting on their laurels, even in the era of the coronavirus. Here are updates on what the five winners have been doing. —Peter J. Brennan Avatar Partners City of Hope Shortly after Marlo Brooke won the Busi- (AR) quality assurance solution for the U.S. As president of the City of Hope Orange employees down from Duarte. Area univer- ness Journal’s award for co-founding Hunt- Navy for aircraft wiring maintenance for the County, Annette Walker is orchestrating a sities could partner with City of Hope. ington Beach-based Avatar Partners Inc., Naval Air Systems Command’s Boeing V- $1 billion project to build one of the biggest, While the larger campus near the Orange she was accepted into the Forbes Technol- 22 Osprey aircraft. and scientifically advanced, cancer research County Great Park is being built, Walker in ogy Council, an invitation-only community Then the Air Force is using Avatar’s solu- centers in the world. -

2015 Annual Report
ANNUAL REPORT 2015 MARCH 2016 TO OUR SHAREHOLDERS ALEX GORSKY Chairman, Board of Directors and Chief Executive Officer This year at Johnson & Johnson, we are proud this aligned with our values. Our Board of WRITTEN OVER to celebrate 130 years of helping people Directors engages in a formal review of 70 YEARS AGO, everywhere live longer, healthier and happier our strategic plans, and provides regular OUR CREDO lives. As I reflect on our heritage and consider guidance to ensure our strategy will continue UNITES & our future, I am optimistic and confident in the creating better outcomes for the patients INSPIRES THE long-term potential for our business. and customers we serve, while also creating EMPLOYEES long-term value for our shareholders. OF JOHNSON We manage our business using a strategic & JOHNSON. framework that begins with Our Credo. Written OUR STRATEGIES ARE BASED ON over 70 years ago, it unites and inspires the OUR BROAD AND DEEP KNOWLEDGE employees of Johnson & Johnson. It reminds OF THE HEALTH CARE LANDSCAPE us that our first responsibility is to the patients, IN WHICH WE OPERATE. customers and health care professionals who For 130 years, our company has been use our products, and it compels us to deliver driving breakthrough innovation in health on our responsibilities to our employees, care – from revolutionizing wound care in communities and shareholders. the 1880s to developing cures, vaccines and treatments for some of today’s most Our strategic framework positions us well pressing diseases in the world. We are acutely to continue our leadership in the markets in aware of the need to evaluate our business which we compete through a set of strategic against the changing health care environment principles: we are broadly based in human and to challenge ourselves based on the health care, our focus is on managing for the results we deliver. -

Mcneil Consumer : Mdl No
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA IN RE: MCNEIL CONSUMER : MDL NO. 2190 HEALTHCARE, ET AL., MARKETING : AND SALES PRACTICES LITIGATION : : Applies to: : ALL ACTIONS : MEMORANDUM McLaughlin, J. July 13, 2012 This multidistrict litigation arises out of quality control problems at the defendants’ facility manufacturing over- the-counter healthcare products in Fort Washington, Pennsylvania, which led to a series of recalls of those products. The named plaintiffs assert claims for economic loss on behalf of a putative nationwide class against Johnson & Johnson (“J&J”), McNeil Consumer Healthcare (“McNeil”), and four of their executives. The plaintiffs allege that they overpaid for the defendants’ products as a result of the recalls and the defendants’ scheme to conceal or downplay the scope of the quality control problems. The defendants, who have offered a coupon or cash refund to consumers who purchased recalled drugs, have moved to dismiss the operative complaint, and assert that the named plaintiffs lack constitutional standing and have not met the applicable pleading standard. The Court will grant the defendants’ motion because the plaintiffs have not pled facts that show a cognizable injury in fact, which is required to confer Article III standing. I. Procedural Background This litigation resulted from the consolidation of ten individual actions filed around the country. Haviland v. McNeil Consumer Healthcare, No. 10-2195, was filed in this Court on May 12, 2010, asserting economic injuries arising out of the April 30, 2010 recall of over-the-counter children’s drugs by McNeil, a part of the J&J “Family of Companies.” Eight additional cases, also arising out of the April 2010 recall, were filed in district courts around the country.1 All cases asserted claims for economic injury only, with the exception of Rivera v. -

Approved Prenatal Medications Pain Medications • Tylenol
Approved Prenatal Medications Pain Medications Tylenol (acetaminophen) for minor aches and pains, headaches. (Do not use: Aspirin, Motrin, Advil, Aleve, Ibuprofen.) Coughs/Colds Robitussin (Cough) Robitussin DM (non-productive cough) DO NOT USE TILL OVER 12 WEEKS Secrets and Vicks Throat Lozenges Mucinex Sore Throat Chloraseptic spray Saline Gargle Sucrets and Vicks Throat Lozenges Antihistamines/Allergies Zyrtec Claritin Benadryl Dimetapp Insomnia Benadryl Unison Hemorrhoids Preparation H Tucks Anusol Diarrhea Imodium (1-2 doses- if it persists please notify the office) BRAT diet (bananas, rice, applesauce, toast) Lice RID (only!) DO NOT USE Kwell Itching Benadryl Calamine or Caladryl Lotion Hydrocortisone cream Heartburn, Indigestion, Gas Tums Gas-X Mylanta Pepcid Maalox Zantac *DO NOT USE PEPTO BISMOL- it contains aspirin Decongestants Sudafed Robitussin CF- Only if over 12 weeks Tavist D Ocean Mist Nasal Spray (saline solutions) Nausea Small Frequent Meals Ginger Ale Vitamin B6 Sea Bands Yeast Infections Monistat Mycolog Gyne-lotrimin Toothache Orajel May see dentists, have cavity filled using Novocain or lidocaine, have x-rays with double lead shield, may have antibiotics in the Penicillin family (penicillin, amoxicillin) Sweetners- all should be consumed in moderation with water being consumed more frequently Nutrisweet (aspartame) Equal (aspartame) Splenda (sucralose) Sweet’n Low (saccharin) *note avoid aspartame if you have phenylketonuria (PKU) Constipation Colace Fibercon Citrucel Senokot Metamucil Milk of Magnesia Fiberall Miralax Eczema Hydrocortisone Cream Medications to AVOID Accurate Lithium Paxil Ciprofloxacin Tetracycline Coumadin Other Chemicals to AVOID Cigarettes Alcohol Recreational Drugs: marijuana, cocaine, ecstasy, heroin . -

Johnson & Johnson Reports 2010 Second-Quarter Results
Johnson & Johnson Reports 2010 Second-Quarter Results: Sales of $15.3 Billion Increased 0.6% Versus 2009 Second-Quarter; EPS was $1.23 Excluding Special Items, 2010 Second-Quarter EPS was $1.21, an increase of 5.2%* NEW BRUNSWICK, N.J., July 20, 2010 /PRNewswire via COMTEX News Network/ -- Johnson & Johnson (NYSE: JNJ) today announced sales of $15.3 billion for the second quarter of 2010, an increase of 0.6% as compared to the second quarter of 2009. Operational results increased 0.1% and the positive impact of currency was 0.5%. Domestic sales declined 2.8%, while international sales increased 4.1%, reflecting operational growth of 3.0% and a positive currency impact of 1.1%. Net earnings and diluted earnings per share for the second quarter of 2010 were $3.4 billion and $1.23, respectively. Second- quarter 2010 net earnings included an after-tax gain of $67 million representing the net impact of litigation matters. Excluding this special item, net earnings for the current quarter were $3.4 billion and diluted earnings per share were $1.21, representing increases of 5.4% and 5.2%, respectively, as compared to the same period in 2009.* The Company updated its earnings guidance for full-year 2010 to $4.65 - $4.75 per share, which excludes the impact of special items. The Company's guidance now reflects the impact of the voluntary recalls announced earlier this year of certain over-the-counter medicines and the suspension of manufacturing at the McNeil Consumer Healthcare facility in Fort Washington, Pa., as well as unfavorable changes in foreign currency exchange rates. -
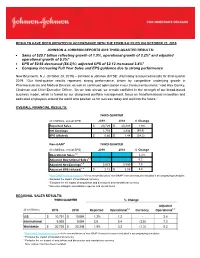
Sales of $20.7 Billion Reflecting Growth of 1.9%, Operational Growth of 3.2
RESULTS HAVE BEEN UPDATED IN ACCORDANCE WITH THE FORM 8-K FILED ON OCTOBER 23, 2019 JOHNSON & JOHNSON REPORTS 2019 THIRD-QUARTER RESULTS: • Sales of $20.7 billion reflecting growth of 1.9%, operational growth of 3.2%* and adjusted operational growth of 5.2%* • EPS of $0.66 decreased (54.2)%; adjusted EPS of $2.12 increased 3.4%* • Company increasing Full Year Sales and EPS guidance due to strong performance New Brunswick, N.J. (October 23, 2019) – Johnson & Johnson (NYSE: JNJ) today announced results for third-quarter 2019. “Our third-quarter results represent strong performance, driven by competitive underlying growth in Pharmaceuticals and Medical Devices, as well as continued optimization in our Consumer business,” said Alex Gorsky, Chairman and Chief Executive Officer. “As we look ahead, we remain confident in the strength of our broad-based business model, which is fueled by our disciplined portfolio management, focus on transformational innovation and dedicated employees around the world who position us for success today and well into the future.” OVERALL FINANCIAL RESULTS: THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Reported Sales $ 20,729 $ 20,348 1.9% Net Earnings 1,753 3,934 (55.4) EPS (diluted) $ 0.66 $ 1.44 (54.2) Non-GAAP* THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Operational Sales1,2 3.2% Adjusted Operational Sales1,3 5.2 Adjusted Net Earnings1,4 5,672 5,590 1.5 Adjusted EPS (diluted)1,4 $ 2.12 $ 2.05 3.4 1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures -

Department of Public Health 105 Cmr 720.000
105 CMR: DEPARTMENT OF PUBLIC HEALTH 105 CMR 720.000: LIST OF INTERCHANGEABLE DRUG PRODUCTS Section 720.001: Purpose 720.002: Citation 720.010: Scope and Application 720.020: Definitions Standards 720:040: Commission Review of Relevant Drug Products 720.050: List of Interchangeable Drug Products 720.060: Drug Products Excluded 720.070: Amendments to the Massachusetts List of Interchangeable Drugs Procedures for Amending List of Interchangeable Drug Products 720.080: Procedures for Amending the Massachusetts List of Interchangeable Drugs 720.081: Petition to Amend List of Interchangeable Drug Products 720.082: Commission Review of Petition 720.083: Notice of Public Comment Period 720.084: Commission Recommendation of Amendments to Department 720.090: Department Adoption of Amendments 720.100: Severability 720.200: Appendix A: List of Interchangeable Drugs 720.001: Purpose The purpose of 105 CMR 720.000 is to establish a drug formulary, or list of interchangeable drug products, for use by physicians, other practitioners, and pharmacists licensed to practice within the commonwealth, so that consumers of prescription drug products may realize cost savings by buying less expensive, safe drug products. 720.002: Citation 105 CMR 720.000 shall be known as the 105 CMR 720.000: Massachusetts List of Interchangeable Drug Products. 720.010: Scope and Application 105 CMR 720.000 establishes the list of interchangeable drug products from which a pharmacist must interchange a reasonably available less expensive drug product than that written, when a prescription written by a practitioner indicates "interchange". 105 CMR 720.000 also establishes criteria and procedures for inclusion of drug products on this list. -

Nevada Medicaid Formulary
Nevada Medicaid-Approved Preferred Drug List Effective August 15, 2021 Legend In each class, drugs are listed alphabetically by either brand name or generic name. Brand name drug: Uppercase in bold type Generic drug: Lowercase in plain type AL: Age Limit Restrictions DO: Dose Optimization Program GR: Gender Restriction OTC: Over the counter medication available with a prescription. (Prescribers please indicate OTC on the prescription) PA: Prior authorization is required. Prior authorization is the process of obtaining approval of benefits before certain prescriptions are filled. QL: Quantity limits; certain prescription medications have specific quantity limits per prescription or per month. SP: Specialty Pharmacy ST: Step therapy is required. You may need to use one medication before benefits for the use of another medication can be authorized. Drug Name Reference Notes *ADHD/ANTI-NARCOLEPSY/ANTI- OBESITY/ANOREXIANTS* *ADHD AGENT - SELECTIVE ALPHA ADRENERGIC AGONISTS*** clonidine hcl er oral tablet extended release Kapvay AL; QL 12 hour *ADHD AGENT - SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR*** atomoxetine hcl oral capsule Strattera DO; AL; QL *AMPHETAMINE MIXTURES*** amphetamine-dextroamphet er oral capsule extended release 24 hour 10 mg, 15 mg, 5 Adderall XR DO; AL; QL mg amphetamine-dextroamphet er oral capsule extended release 24 hour 20 mg, 25 mg, 30 Adderall XR AL; QL mg amphetamine-dextroamphetamine oral Adderall DO; AL; QL tablet 10 mg, 12.5 mg, 15 mg, 5 mg, 7.5 mg amphetamine-dextroamphetamine oral Adderall AL tablet 20 mg, -

Exhibitor & Sponsor Program
Exhibitor & Sponsor Program www.AtlanticDermConference.org @AtlanticDermADC The Philadelphia Dermatological Society and the Atlantic Derm Conference are pleased to acknowledge and express our sincere appreciation for our corporate sponsors, exhibitors, and commercial supporters. Disclosure of Commercial Support | This activity is supported by an educational donation provided by Amgen, an educational grant provided by Galderma Laboratories, LP, and an educational grant provided by Valeant Dermatology. SPONSORS DIAMOND PLATINUM GOLD SILVER Pharmaceuticals North America LLC www.AtlanticDermConference.org | Page 1 " !"!%! "%#%! !! !#! !% "!! #!!#%$ %"!"! ! !!!! " !%"!! '!!!&! ! "! ' !!! !!"!% "%# ! ( ! # ' % "& ## #!!#" #"$" Exhibitors Exhibitors 3Gen, Inc. | 31521 Rancho Viejo Road, #104, San Juan Capistrano, CA 92675 | 949.481.6384 | www.dermlite.com Table #15 ADDRESSING 3Gen manufactures the DermLite brand of skin imaging devices. THE TOUGHEST AbbVie | 1 N. Waukegan Road, North Chicago, IL 60064 | 847.937.7390 | abbvie.com | www.psoriasis.com Table #1 & 2 AbbVie is a global, research-based biopharmaceutical company which combines the focus of a leading-edge biotech with DERMATOLOGY the expertise and structure of a long-established pharmaceutical leader. AbbVie is committed to using unique approaches to innovation to develop and market advanced therapies that address some of the world’s most complex, serious diseases. CHALLENGES Actelion Pharmaceuticals US, Inc. | 5000 Shoreline Ct., South San Francisco, -

Tips for Managing Treatment-Related Rash and Dry Skin
RASH Tips for Managing Treatment-Related Rash and Dry Skin Presented by Stewart B. Fleishman, MD Continuum Cancer Centers of New York: Beth Israel & St. Luke’s-Roosevelt Lindy P. Fox, MD University of California San Francisco David H. Garfield, MD University of Colorado Comprehensive Cancer Center Carol S. Viele, RN, MS University of California San Francisco Carolyn Messner, DSW CancerCare Learn about: • Effects of targeted treatments on the skin • Managing rashes and dry skin • Treating nail conditions • Your support team Help and Hope CancerCare is a national nonprofit organization that provides free support services to anyone affected by cancer: people with cancer, caregivers, children, loved ones, and the bereaved. CancerCare programs—including counseling and support groups, education, financial assistance, and practical help—are provided by professional oncology social workers and are completely free of charge. Founded in 1944, CancerCare provided individual help to more than 100,000 people last year and had more than 1 million unique visitors to our websites. For more information, call 1-800-813-HOPE (4673) or visit www.cancercare.org. Contacting CancerCare National Office Administration CancerCare Tel: 212-712-8400 The material presented in this patient booklet is provided for your general 275 Seventh Avenue Fax: 212-712-8495 information only. It is not intended as medical advice and should not be relied New York, NY 10001 Email: [email protected] upon as a substitute for consultations with qualified health professionals who Email: [email protected] Website: www.cancercare.org are aware of your specific situation. We encourage you to take information and Services questions back to your individual health care provider as a way of creating a Tel: 212-712-8080 dialogue and partnership about your cancer and your treatment. -

2019 SMDP Biotech Training Agenda & Scholar Bios
2019 SMDP Biotech 1-5 June PHILADELPHIA www.icpdprograms.org 2019 Scientist Mentoring & Diversity Program for biotechnology (SMDP Biotech) www.icpdprograms.org Training Session June 1-6, 2019 in Philadelphia, PA about the program who attends: the one-year career mentoring program pairs ethnically diverse post-baccalaureate students, graduate students and post-doctoral researchers with industry mentors who work at biotechnology and consumer healthcare companies. With their mentors, SMDP Biotech Scholars attend a 5-day training session to learn about career opportunities in industry and receive career coaching. SMDP Scholars and Mentors also attend The BIO International Convention. how to dress, what to bring: business attire with comfortable shoes. Scholars, bring 100 business cards and 10 copies of your resume. Mentors will need business cards too. Informal dinner where to go: SMDP training Scholars and Mentors you’re Host Hotel session day 1 already on the guest list, we Mentors you will arrange the bus leaves the leave J&J Consumer at 5:15pm your own accommodation host hotel at 6:30am Ladder 15, 1528 Sansom Street, Scholars a shared room Scholars you will be Philadelphia, PA 19102 reservation has already been introduced to your made for you at this location: Mentors at the Reading Terminal MarKet Philadelphia 201, 201 N. 17th training session The bus leaves from the SMDP J&J Consumer, Street, Philadelphia, PA 19103 training session at 11:30am 199 Grandview Road, Corner of North 12th Street “Celebration of Mentoring Skillman, NJ 08558 and Arch Street (South Bldg. SK1912) & Diversity” reception ($10 will be provided) (Saturday, June 1st at 6pm) SMDP training The BIO Intl Convention Scholars and Mentors you’re session day 2 registration information will be already on the guest list the bus leaves the provided during the SMDP The Pyramid Club, host hotel at 7am training session. -

Creative Director 561.714.1585 Andy Mathurin
Hello, I am: Looking for role as: Let’s Connect: Andy Mathurin Creative Director 561.714.1585 Associate Creative Director, [email protected] Brand Strategist New York What I do: PROFILE Brand Strategy Over 8 years leading branding and marketing concepts with career spanning 360 campaigns, broadcast, print, OOH, social, digital, video and experiential Identity Design across major worldwide brands. Energetic and a creative visionary offering Storytelling demonstrated expertise in all aspects of branding and strategy, with core focus Thought Leader on delivering business results. Team Builder Marketing WINS Project Management Successes have included winning several accounts for new business, resulting in agency being awarded Global Agency of the Year by AdAge & Adweek. Toolkit: AOR: Havas - Vascepa 2019, Havas - Alcon 2019, J3 - OGX 2018, UM - H&M 2017, UM - Sony 2017, BMW 2016, UM - Coca Cola 2016 Sketch XD Microsoft Office PROFESSIONAL EXPERIENCE Avocode 03.18 HAVAS | Manhattan, NY Photoshop Present Associate Creative Director Strategic partner, focusing on brand equity for healthcare and InDesign pharmaceutical brands. Responsible for new business and leading creative Illustration team/studio across all digital platforms (specializing in social). After Effects Brands include: Zicam, Alcon, GSK, NUCALA, TRELEGY, & ANORO. 09.14 Universal McCann Worldwide | Manhattan, NY 03.18 Personality: Associate Creative Director + Senior Art Director Hands-on leader in identity design. Champion participant in winning new Human business pitches. Guiding and advising clients on high-level executions. Confident Brands include: Sony Pictures, GoPro, McCormick, BMW... and more. Effective Communicator - Demonstrate strong leadership overseeing staff in the day-to-day projects Motivational Honest with Integrity - Deliver against demanding brand objectives and develop creative that Empathetic exceeds business needs, managing projects from concept through completion: timelines, budgets, schedules, etc.