Benzene in Sunscreen and After-Sun Care Products
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety of Oxybenzone: Putting Numbers Table
Safety of Oxybenzone: Putting Numbers Table. Years of Daily Sunscreen Application Required by an Average US Woman to Reach Systemic Levels Into Perspective of Oxybenzone per Unit of Body Mass Equivalent to Those Given to Immature Rats10 xybenzone, an organic UV-B and short-wave UV-A filter, has been available for over 40 years1; Scenario Scenario Scenario it is widely used in sunscreens and other con- Characteristic 1 2 3 O 2 sumer products in the United States. The Centers for Dis- Body surface area covered, % 100 100 25 ease Control and Prevention has estimated the preva- Application dose, mg/cm2/d211 lence of oxybenzone exposure in the general US Application amount required, 30.00 15.00 3.75 population to be 96.8%.3 In the past few years, oxyben- mL/d Time required, y 34.6 69.3 277.0 zone has received increasing attention as a potentially harmful compound. Initial concerns arose when a re- port demonstrated systemic absorption of oxybenzone in humans at a rate of 1% to 2% after topical applica- For the purposes of this estimation, a generous in-use dose of 1 mg/cm2 was used,13-16 and it was assumed that cov- tion.4 Similar or higher rates of cutaneous absorption in 5-9 erage of 25% of the human BSA was limited to the face, human subjects have been observed. The potential for neck, hands, and arms.17 biological effects, however, were first published in a study 10 by Schlumpf et al demonstrating uterotropic effects in Results. The numbers of years of daily application re- immature rats after oral administration of oxybenzone; quired to obtain the equivalent amount of sunscreen un- it should be noted that the estrogenic effect detected was der the 3 scenarios are listed in the Table. -

GAO-18-61, SUNSCREEN: FDA Reviewed Applications For
United States Government Accountability Office Report to Congressional Committees November 2017 SUNSCREEN FDA Reviewed Applications for Additional Active Ingredients and Determined More Data Needed GAO-18-61 November 2017 SUNSCREEN FDA Reviewed Applications for Additional Active Ingredients and Determined More Data Needed Highlights of GAO-18-61, a report to congressional committees Why GAO Did This Study What GAO Found Using sunscreen as directed with other The Food and Drug Administration (FDA), within the Department of Health and sun protective measures may help Human Services, implemented requirements for reviewing applications for reduce the risk of skin cancer—the sunscreen active ingredients within time frames set by the Sunscreen Innovation most common form of cancer in the Act, which was enacted in November 2014. For example, the agency issued a United States. In the United States, guidance document on safety and effectiveness testing in November 2016. sunscreen is considered an over-the- counter drug, which is a drug available As of August 2017, all applications for sunscreen active ingredients remain to consumers without a prescription. pending after the agency determined more safety and effectiveness data are Some sunscreen active ingredients not needed. By February 2015, FDA completed its initial review of the safety and currently marketed in the United States effectiveness data for each of the eight pending applications, as required by the have been available in products in act. FDA concluded that additional data are needed to determine that the other countries for more than a ingredients are generally recognized as safe and effective (GRASE), which is decade. Companies that manufacture needed so that products using the ingredients can subsequently be marketed in some of these ingredients have sought the United States without FDA’s premarket approval. -

Women in Business Awards Luncheon at the Hotel Irvine, Where Aston Martin Americas President Laura Schwab Delivered the Keynote Address
10.5.20 SR_WIB.qxp_Layout 1 10/2/20 12:14 PM Page 29 WOMEN IN BUSINESS NOMINEES START ON PAGE B-60 INSIDE 2019 WINNERS GO BIG IN IRVINE, LAND NEW PARTNERS, INVESTMENTS PAGE 30 PRESENTED BY DIAMOND SPONSOR PLATINUM SPONSORS GOLD SPONSOR SILVER SPONSORS 10.5.20 SR_WIB.qxp_Layout 1 10/2/20 1:36 PM Page 30 30 ORANGE COUNTY BUSINESS JOURNAL www.ocbj.com OCTOBER 5, 2020 Winning Execs Don’t Rest on Their Laurels $1B Cancer Center Underway; Military Wins; Spanish Drug Investment Orange County’s business community last year celebrated the Business Journal’s 25th annual Women in Business Awards luncheon at the Hotel Irvine, where Aston Martin Americas President Laura Schwab delivered the keynote address. The winners, selected from 200 nominees, have not been resting on their laurels, even in the era of the coronavirus. Here are updates on what the five winners have been doing. —Peter J. Brennan Avatar Partners City of Hope Shortly after Marlo Brooke won the Busi- (AR) quality assurance solution for the U.S. As president of the City of Hope Orange employees down from Duarte. Area univer- ness Journal’s award for co-founding Hunt- Navy for aircraft wiring maintenance for the County, Annette Walker is orchestrating a sities could partner with City of Hope. ington Beach-based Avatar Partners Inc., Naval Air Systems Command’s Boeing V- $1 billion project to build one of the biggest, While the larger campus near the Orange she was accepted into the Forbes Technol- 22 Osprey aircraft. and scientifically advanced, cancer research County Great Park is being built, Walker in ogy Council, an invitation-only community Then the Air Force is using Avatar’s solu- centers in the world. -

Two-Step Semi-Microscale Preparation of a Cinnamate Ester Sunscreen Analog W
In the Laboratory edited by The Microscale Laboratory R. David Crouch Dickinson College Carlisle, PA 17013-2896 Two-Step Semi-Microscale Preparation of a Cinnamate Ester Sunscreen Analog W Ryan G. Stabile and Andrew P. Dicks* Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario, Canada, M5S 3H6; *[email protected] Several laboratory experiments concerning the physical A two-step procedure using 4-methoxybenzaldehyde as properties of sunscreens have appeared in this Journal dur- the starting material (Scheme I) synthesizes ethyl trans-4- ing the last decade (1–5). These include quantification of methoxycinnamate 1. In the first laboratory session students commercial formulations by liquid chromatography (1) and are exposed to an important carbon–carbon bond forming ultraviolet (UV) spectrophotometry (2–4). The photochem- condensation reaction. The Verley–Doebner modification of istry of sunscreens has also been reviewed (6). Significantly, the Knoevenagel condensation (11) affords facile synthesis a student procedure focusing on multistep sunscreen synthesis of trans-4-methoxycinnamic acid, which is isolated and char- and spectroscopic analysis has not, to our knowledge, been acterized. During the second period, esterification is effected reported. Given the current high profile nature of skin can- by a cesium base mediated O-alkylation approach. This ex- cer (7) and media attention towards sunscreens, we designed emplifies the so-called “cesium effect” (12) and the useful- a two-step synthetic pathway towards an analog of a com- ness of cesium carboxylates as nucleophiles in SN2 reactions. mercially available UV light blocker. This methodology is in- In addition to stimulating class enthusiasm towards syn- corporated into a third-year undergraduate organic synthesis thetic chemistry, this experiment impresses many organic course at the University of Toronto. -

Ultraviolet Radiation, Aging and the Skin: Prevention of Damage by Topical Camp Manipulation
Molecules 2014, 19, 6202-6219; doi:10.3390/molecules19056202 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Ultraviolet Radiation, Aging and the Skin: Prevention of Damage by Topical cAMP Manipulation Alexandra Amaro-Ortiz 1, Betty Yan 1 and John A. D’Orazio 1,2,* 1 The Graduate Center for Toxicology, the Markey Cancer Center and the Department of Pediatrics, University of Kentucky College of Medicine, 800 Rose Street, Lexington, KY 40536, USA 2 Markey Cancer Center, University of Kentucky College of Medicine, Combs Research Building 204, 800 Rose Street, Lexington, KY 40536-0096, USA * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-859-323-6238; Fax: +1-859-257-8940. Received: 26 April 2014; in revised form: 8 May 2014 / Accepted: 13 May 2014 / Published: 15 May 2014 Abstract: Being the largest and most visible organ of the body and heavily influenced by environmental factors, skin is ideal to study the long-term effects of aging. Throughout our lifetime, we accumulate damage generated by UV radiation. UV causes inflammation, immune changes, physical changes, impaired wound healing and DNA damage that promotes cellular senescence and carcinogenesis. Melanoma is the deadliest form of skin cancer and among the malignancies of highest increasing incidence over the last several decades. Melanoma incidence is directly related to age, with highest rates in individuals over the age of 55 years, making it a clear age-related disease. In this review, we will focus on UV-induced carcinogenesis and photo aging along with natural protective mechanisms that reduce amount of “realized” solar radiation dose and UV-induced injury. -

Photophysics and Skin Penetration of Active Agents in a Commercial Sunscreen and Insect Repellent
PHOTOPHYSICS AND SKIN PENETRATION OF ACTIVE AGENTS IN A COMMERCIAL SUNSCREEN AND INSECT REPELLENT by DONALD PRETTYPAUL A Dissertation submitted to the Graduate School-Newark Rutgers, The State University of New Jersey In partial fulfillment of the requirements for the degree of Doctor of Philosophy Graduate Program in Chemistry written under the direction of Professor Richard Mendelsohn Professor Piotr Piotrowiak and approved by Newark, New Jersey October 2018 ©2018 Donald Prettypaul ALL RIGHTS RESERVED ABSTRACT OF DISSERTATION PHOTOPHYSICS AND SKIN PENETRATION OF ACTIVE AGENTS IN A COMMERCIAL SUNSCREEN AND INSECT REPELLENT By DONALD PRETTYPAUL Dissertation co-Directors: Professor Richard Mendelsohn Professor Piotr Piotrowiak This dissertation is focused on active agents in commercial sunscreen and insect repellent products. It consists of two parts, the first focusing on the photophysics of a sunscreen active agent and the second on the permeation and spatial distribution of the sunscreen active and an insect repellent active when these agents are applied to ex-vivo human skin. In the photochemistry study, ultrafast spectroscopy was used to study the excited state dynamics of the sunscreen molecule, Bemotrizinol. The work focused on the dissipation rates of the electronic excitation energy in different solvents. To complement the results from time-resolved femtosecond spectroscopy, Hartree- Fock UH/UHF 6-31G* calculations were used to characterize the ground and excited states potential energy surfaces. The results indicate that the excited state deactivation pathway follows a proton coupled electron transfer process which ii proceeds via a concerted mechanism. The dependencies on solvent polarity, viscosity, and H/D isotope effects, were investigated. Sunscreen products have been developed to protect skin from ultraviolet (UV) radiation; to achieve adequate protection, the sunscreen must be evenly applied and remain on the surface of the skin. -

Technical Appendix to the Sunscreen Proposed Rule
DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Technical Appendix to the Sunscreen Proposed Rule 1 Table of Contents I. Overview ................................................................................................................................. 3 A. Definitions ........................................................................................................................ 3 II. Sunscreen Product Counts ...................................................................................................... 4 A. Sunscreen Product Search ................................................................................................ 4 B. Private Label Products ..................................................................................................... 6 C. Sunscreen Firms and Manufacturers ................................................................................ 7 D. Number of Relabeled and Reformulated Units ................................................................ 9 1. Relabeled Units............................................................................................................. 9 2. Reformulated Units....................................................................................................... 9 III. Sales by Product ................................................................................................................. 10 A. Total Market Sales ........................................................................................................ -

Johnson & Johnson Reports 2010 Second-Quarter Results
Johnson & Johnson Reports 2010 Second-Quarter Results: Sales of $15.3 Billion Increased 0.6% Versus 2009 Second-Quarter; EPS was $1.23 Excluding Special Items, 2010 Second-Quarter EPS was $1.21, an increase of 5.2%* NEW BRUNSWICK, N.J., July 20, 2010 /PRNewswire via COMTEX News Network/ -- Johnson & Johnson (NYSE: JNJ) today announced sales of $15.3 billion for the second quarter of 2010, an increase of 0.6% as compared to the second quarter of 2009. Operational results increased 0.1% and the positive impact of currency was 0.5%. Domestic sales declined 2.8%, while international sales increased 4.1%, reflecting operational growth of 3.0% and a positive currency impact of 1.1%. Net earnings and diluted earnings per share for the second quarter of 2010 were $3.4 billion and $1.23, respectively. Second- quarter 2010 net earnings included an after-tax gain of $67 million representing the net impact of litigation matters. Excluding this special item, net earnings for the current quarter were $3.4 billion and diluted earnings per share were $1.21, representing increases of 5.4% and 5.2%, respectively, as compared to the same period in 2009.* The Company updated its earnings guidance for full-year 2010 to $4.65 - $4.75 per share, which excludes the impact of special items. The Company's guidance now reflects the impact of the voluntary recalls announced earlier this year of certain over-the-counter medicines and the suspension of manufacturing at the McNeil Consumer Healthcare facility in Fort Washington, Pa., as well as unfavorable changes in foreign currency exchange rates. -

Sunscreen Faqs
Sunscreen FAQs What type of sunscreen should I use? There are two main types of sunscreen ingredients: physical sun blockers and chemical sunscreens. Physical sunscreens prevent ultraviolet light from reaching your skin, and contain either zinc oxide or titanium dioxide. Physical sunscreens protect against UVA and UVB damage. Physical sunscreens are preferred for patients with sensitive skin or children. Chemical sunscreens rely on an interaction between the sun and the chemical to protect your skin. Examples of such chemicals are avobenzone, octinoxate, homosalate, padimate O, and many others. Chemical sunscreens can protect against UVA, UVB, or both types of damage, depending on which ingredient is used. Read the label to ensure coverage for both UVA and UVB exposure. There are new ingredients being developed that take chemical sunscreens and stabilize them to prolong their activity (i.e., Helioplex, Mexoryl). Does the SPF really matter? In laboratory testing, there are minor differences between SPF 15 and SPF 30 or greater products. However, that data is based on using 1 oz. (30 gm.) of sunscreen for each full body application (the size of a shot glass). Most people use far less in real-life settings. For that reason, we recommend an SPF of at least 30. It is common to find good sunscreens with SPF ranging from 45-70. To maintain protection, reapply sunscreen every 1-3 hours, depending on sweating, water exposure, and sun intensity. Is sunscreen alone enough to protect my skin in the sun? In some cases, yes. However, if you are in the sun for longer periods on a regular basis (such as gardening, golfing, boating, sports), it may be better to add sun protective clothing or habits. -
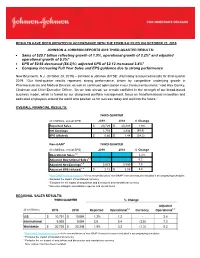
Sales of $20.7 Billion Reflecting Growth of 1.9%, Operational Growth of 3.2
RESULTS HAVE BEEN UPDATED IN ACCORDANCE WITH THE FORM 8-K FILED ON OCTOBER 23, 2019 JOHNSON & JOHNSON REPORTS 2019 THIRD-QUARTER RESULTS: • Sales of $20.7 billion reflecting growth of 1.9%, operational growth of 3.2%* and adjusted operational growth of 5.2%* • EPS of $0.66 decreased (54.2)%; adjusted EPS of $2.12 increased 3.4%* • Company increasing Full Year Sales and EPS guidance due to strong performance New Brunswick, N.J. (October 23, 2019) – Johnson & Johnson (NYSE: JNJ) today announced results for third-quarter 2019. “Our third-quarter results represent strong performance, driven by competitive underlying growth in Pharmaceuticals and Medical Devices, as well as continued optimization in our Consumer business,” said Alex Gorsky, Chairman and Chief Executive Officer. “As we look ahead, we remain confident in the strength of our broad-based business model, which is fueled by our disciplined portfolio management, focus on transformational innovation and dedicated employees around the world who position us for success today and well into the future.” OVERALL FINANCIAL RESULTS: THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Reported Sales $ 20,729 $ 20,348 1.9% Net Earnings 1,753 3,934 (55.4) EPS (diluted) $ 0.66 $ 1.44 (54.2) Non-GAAP* THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Operational Sales1,2 3.2% Adjusted Operational Sales1,3 5.2 Adjusted Net Earnings1,4 5,672 5,590 1.5 Adjusted EPS (diluted)1,4 $ 2.12 $ 2.05 3.4 1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures -

FDA Proposes Sunscreen Regulation Changes February 2019
FDA Proposes Sunscreen Regulation Changes February 2019 The U.S. Food and Drug Administration (FDA) regulates sunscreens to ensure they meet safety and eectiveness standards. To improve the quality, safety, and eectiveness of sunscreens, FDA issued a proposed rule that describes updated proposed requirements for sunscreens. Given the recognized public health benets of sunscreen use, Americans should continue to use broad spectrum sunscreen with SPF 15 or higher with other sun protective measures as this important rulemaking eort moves forward. Highlights of FDA’s Proposals Sunscreen active ingredient safety and eectiveness Two ingredients (zinc oxide and titanium dioxide) are proposed to be safe and eective for sunscreen use and two (aminobenzoic acid (PABA) and trolamine salicylate) are 1 proposed as not safe and eective for sunscreen use. FDA proposes that it needs more safety information for the remaining 12 sunscreen ingredients (cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, padimate O, sulisobenzone, oxybenzone, avobenzone). New proposed sun protection factor Sunscreen dosage forms (SPF) and broad spectrum Sunscreen sprays, oils, lotions, creams, gels, butters, pastes, ointments, and sticks are requirements 2 proposed as safe and eective. FDA 3 • Raise the maximum proposed labeled SPF proposes that it needs more data for from SPF 50+ to SPF 60+ sunscreen powders. • Require any sunscreen SPF 15 or higher to be broad spectrum • Require for all broad spectrum products SPF 15 and above, as SPF increases, broad spectrum protection increases New proposed label requirements • Include alphabetical listing of active ingredients on the front panel • Require sunscreens with SPF below 15 to include “See Skin Cancer/Skin Aging alert” on the front panel 4 • Require font and placement changes to ensure SPF, broad spectrum, and water resistance statements stand out Sunscreen-insect repellent combination 5 products proposed not safe and eective www.fda.gov. -

Department of Public Health 105 Cmr 720.000
105 CMR: DEPARTMENT OF PUBLIC HEALTH 105 CMR 720.000: LIST OF INTERCHANGEABLE DRUG PRODUCTS Section 720.001: Purpose 720.002: Citation 720.010: Scope and Application 720.020: Definitions Standards 720:040: Commission Review of Relevant Drug Products 720.050: List of Interchangeable Drug Products 720.060: Drug Products Excluded 720.070: Amendments to the Massachusetts List of Interchangeable Drugs Procedures for Amending List of Interchangeable Drug Products 720.080: Procedures for Amending the Massachusetts List of Interchangeable Drugs 720.081: Petition to Amend List of Interchangeable Drug Products 720.082: Commission Review of Petition 720.083: Notice of Public Comment Period 720.084: Commission Recommendation of Amendments to Department 720.090: Department Adoption of Amendments 720.100: Severability 720.200: Appendix A: List of Interchangeable Drugs 720.001: Purpose The purpose of 105 CMR 720.000 is to establish a drug formulary, or list of interchangeable drug products, for use by physicians, other practitioners, and pharmacists licensed to practice within the commonwealth, so that consumers of prescription drug products may realize cost savings by buying less expensive, safe drug products. 720.002: Citation 105 CMR 720.000 shall be known as the 105 CMR 720.000: Massachusetts List of Interchangeable Drug Products. 720.010: Scope and Application 105 CMR 720.000 establishes the list of interchangeable drug products from which a pharmacist must interchange a reasonably available less expensive drug product than that written, when a prescription written by a practitioner indicates "interchange". 105 CMR 720.000 also establishes criteria and procedures for inclusion of drug products on this list.