GAO-18-61, SUNSCREEN: FDA Reviewed Applications For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety of Oxybenzone: Putting Numbers Table
Safety of Oxybenzone: Putting Numbers Table. Years of Daily Sunscreen Application Required by an Average US Woman to Reach Systemic Levels Into Perspective of Oxybenzone per Unit of Body Mass Equivalent to Those Given to Immature Rats10 xybenzone, an organic UV-B and short-wave UV-A filter, has been available for over 40 years1; Scenario Scenario Scenario it is widely used in sunscreens and other con- Characteristic 1 2 3 O 2 sumer products in the United States. The Centers for Dis- Body surface area covered, % 100 100 25 ease Control and Prevention has estimated the preva- Application dose, mg/cm2/d211 lence of oxybenzone exposure in the general US Application amount required, 30.00 15.00 3.75 population to be 96.8%.3 In the past few years, oxyben- mL/d Time required, y 34.6 69.3 277.0 zone has received increasing attention as a potentially harmful compound. Initial concerns arose when a re- port demonstrated systemic absorption of oxybenzone in humans at a rate of 1% to 2% after topical applica- For the purposes of this estimation, a generous in-use dose of 1 mg/cm2 was used,13-16 and it was assumed that cov- tion.4 Similar or higher rates of cutaneous absorption in 5-9 erage of 25% of the human BSA was limited to the face, human subjects have been observed. The potential for neck, hands, and arms.17 biological effects, however, were first published in a study 10 by Schlumpf et al demonstrating uterotropic effects in Results. The numbers of years of daily application re- immature rats after oral administration of oxybenzone; quired to obtain the equivalent amount of sunscreen un- it should be noted that the estrogenic effect detected was der the 3 scenarios are listed in the Table. -

Senate Passage Media Clips Report September 19, 2014
Senate Passage Media Clips Report September 19, 2014 1. The Hill, September 17, 2014, Senate passes bill to improve sunscreen. By Ramsey Cox 2. TIME, September 17, 2014, Senate Passes Bill for Better Sunscreen. By Alexandra Sifferlin 3. The Daily Caller, September 18, 2014, Senate Bill Passes To End FDA Stranglehold On Sunscreen Innovation. By Jonah Bennet 4. Skin, Inc. September 19, 2014, 2014 Sunscreen Innovation Act Unanimously Passes. 5. Inside Health (Subscription), September 18, 2014, Sunscreen Legislation Backers Seek Swift Enactment After Senate Clears Bill. By Stephanie Beasley 6. Politico Pulse (Subscription), September 18, 2014, Sunscreen bill heads to the House now. 7. Politico Pulse (Subscription), September 18, 2014, HELP approve sunscreen bill. 8. Office of Senator Kay Hagan, September 19, 2014, Bipartisan Sunscreen Innovation Act Cosponsored by Hagan Passes the Senate. 9. Northwest Georgia News, September 18, 2014, Isakson applauds Senate passage of bipartisan bill to help prevent skin cancer. 10. Primer{TBR}, September 18, 2014, Senate Passes Sunscreen Innovation Act to Improve Sunscreen. 11. Smithsonian, September 18, 2014, The US Is Trying to Expedite Sunscreen Innovation. By Rachel Nuwer 12. Cosmetics & Toiletries, September 18, 2014, Sunscreen Innovation Act Passes: What Does it Mean? By Rachel Grahenhofer 13. Office of Senator Mitch McConnell (Press Release), September 18, 2014, McConnell- Sponsored Sunscreen Innovation Act Passes Senate. 14. MarketPlace, September 18, 2014, Your sunscreen is way out of date. By Nancy Marshall-Genzer 37#32748001_v1 15. Earthy Essence, September 18, 2014, 2014 Sunscreen Innovation Act Unanimously Passes. 16. Melanoma Research Alliance, September 17, 2014, MRA Applauds Senate Passage of the Sunscreen Innovation Act. -

Sun Lotion Chemicals As Endocrine Disruptors
HORMONES 2015, 14(1):32-46 Review Sun lotion chemicals as endocrine disruptors Sotirios Maipas, Polyxeni Nicolopoulou-Stamati National and Kapodistrian University of Athens, School of Medicine, First Department of Pathology and Cytology Unit, 1st Pathology Laboratory, Athens, Greece Both authors contributed equally to this work ABSTRACT Ultraviolet solar radiation is a well-known environmental health risk factor and the use of sun lotions is encouraged to achieve protection mainly from skin cancer. Sun lotions are cosmetic commercial products that combine active and inactive ingredients and many of these are associated with health problems, including allergic reactions and endocrine disorders. This review focuses on their ability to cause endocrine and reproductive impairments, with empha- sis laid on the active ingredients (common and less common UV filters). In vitro and in vivo studies have demonstrated their ability to show oestrogenic/anti-oestrogenic and androgenic/ anti-androgenic activity. Many ingredients affect the oestrous cycle, spermatogenesis, sexual behaviour, fertility and other reproductive parameters in experimental animals. Their presence in aquatic environments may reveal a new emerging environmental hazard. Key words: Active ingredients, Endocrine disruptors, Environmental hazard, Reproductive impair- ments, Sun creams, Sun lotions, Sunscreens, UV filters 1. INTRODUCTION ing, but the level of photoprotection is insufficient to prevent the harmful effects of UV radiation.3,4 Ultraviolet (UV) solar radiation is one -
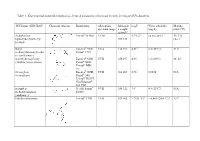
Table 1 - Experimental and Predicted Physical-Chemical Parameters of the Most Recently Investigated UV-Absorbers
Table 1 - Experimental and predicted physical-chemical parameters of the most recently investigated UV-absorbers. INCI name (INN/XAN) Chemical structure Brand name Absorption Molecula LogP Water solubility Melting spectrum range r weight (mg/L) point (°C) (g/mol)4 diethylamino Uvinul® A Plus UVA1 5.7-6.21 <0.01 (20°C) 1 54; 314 hydroxybenzoyl hexyl 397.515 (dec.) 1 benzoate Butyl Eusolex® 9020, UVA 310.393 4.514 2.2 (25°C)4 83.54 methoxydibenzoylmetha Parsol® 1789 ne (avobenzone) 4-methylbenzylidene Eusolex® 6300 UVB 258.397 4.95 1.3 (20°C) 66–68 camphor (enzacamene) Parsol® 5000 Uvinul® MBC 95 Octocrylene Eusolex® OCR, UVB 361.485 6.783 0.00383 N/A (octocrilene) Parsol® 340, Uvinul® N539T, NeoHeliopan® 303 USP isoamyl p- Neo Heliopan® UVB 248.322 3.61 4.9 (25°C)1 N/A methoxycinnamate E1000 (amiloxate) Ethylhexyl triazone Uvinul® T150 UVB 823.092 > 7(20 °C) 6 < 0.001 (20.0 °C) 6 1296 Ethylhexyl Parsol® MCX, UVB 290.403 6.14 0.041 (24 °C and N/A methoxycinnamate Heliopan® New pH 7.1) 4 (octinoxate) Ethylhexyl dimethyl Escalol™ 507 UVB 277.4084 5.774 0.54 (25 °C) 4 N/A PABA (padimate-O) Arlatone 507 Eusolex 6007 benzophenone-3 Eusolex® 4360 UVA2+ UVB 228.247 3.72 3.7 (20°C) 2 62-652 (oxybenzone) bis-ethylhexyloxyphenol Tinosorb® S UVA1+UVB 627.826 12.61 <10-4 80.401 methoxyphenol triazine (bemotrizinol) Phenylbenzimidazole Eusolex® 232 UVA2+ UVB 274.2945 -1.1 (pH 5) > 30% (As sodium N/A sulfonic acid Parsol® HS -2.1 (pH 8)5 or (ensulizole) Neo Heliopan® triethanolammoniu Hydro m salt at 20°C) 5 1 (3) 2 (34) 3 (44) 4 Pubchem 5 SCCP/1056/06 Opinion on phenylbenzimidazole sulfonic acid and its salts 6 BASF safety data sheet Table 2 – In vitro studies for the assessment of skin permeation/penetration of sunscreens. -

Two-Step Semi-Microscale Preparation of a Cinnamate Ester Sunscreen Analog W
In the Laboratory edited by The Microscale Laboratory R. David Crouch Dickinson College Carlisle, PA 17013-2896 Two-Step Semi-Microscale Preparation of a Cinnamate Ester Sunscreen Analog W Ryan G. Stabile and Andrew P. Dicks* Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario, Canada, M5S 3H6; *[email protected] Several laboratory experiments concerning the physical A two-step procedure using 4-methoxybenzaldehyde as properties of sunscreens have appeared in this Journal dur- the starting material (Scheme I) synthesizes ethyl trans-4- ing the last decade (1–5). These include quantification of methoxycinnamate 1. In the first laboratory session students commercial formulations by liquid chromatography (1) and are exposed to an important carbon–carbon bond forming ultraviolet (UV) spectrophotometry (2–4). The photochem- condensation reaction. The Verley–Doebner modification of istry of sunscreens has also been reviewed (6). Significantly, the Knoevenagel condensation (11) affords facile synthesis a student procedure focusing on multistep sunscreen synthesis of trans-4-methoxycinnamic acid, which is isolated and char- and spectroscopic analysis has not, to our knowledge, been acterized. During the second period, esterification is effected reported. Given the current high profile nature of skin can- by a cesium base mediated O-alkylation approach. This ex- cer (7) and media attention towards sunscreens, we designed emplifies the so-called “cesium effect” (12) and the useful- a two-step synthetic pathway towards an analog of a com- ness of cesium carboxylates as nucleophiles in SN2 reactions. mercially available UV light blocker. This methodology is in- In addition to stimulating class enthusiasm towards syn- corporated into a third-year undergraduate organic synthesis thetic chemistry, this experiment impresses many organic course at the University of Toronto. -

Ultraviolet Radiation, Aging and the Skin: Prevention of Damage by Topical Camp Manipulation
Molecules 2014, 19, 6202-6219; doi:10.3390/molecules19056202 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Ultraviolet Radiation, Aging and the Skin: Prevention of Damage by Topical cAMP Manipulation Alexandra Amaro-Ortiz 1, Betty Yan 1 and John A. D’Orazio 1,2,* 1 The Graduate Center for Toxicology, the Markey Cancer Center and the Department of Pediatrics, University of Kentucky College of Medicine, 800 Rose Street, Lexington, KY 40536, USA 2 Markey Cancer Center, University of Kentucky College of Medicine, Combs Research Building 204, 800 Rose Street, Lexington, KY 40536-0096, USA * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-859-323-6238; Fax: +1-859-257-8940. Received: 26 April 2014; in revised form: 8 May 2014 / Accepted: 13 May 2014 / Published: 15 May 2014 Abstract: Being the largest and most visible organ of the body and heavily influenced by environmental factors, skin is ideal to study the long-term effects of aging. Throughout our lifetime, we accumulate damage generated by UV radiation. UV causes inflammation, immune changes, physical changes, impaired wound healing and DNA damage that promotes cellular senescence and carcinogenesis. Melanoma is the deadliest form of skin cancer and among the malignancies of highest increasing incidence over the last several decades. Melanoma incidence is directly related to age, with highest rates in individuals over the age of 55 years, making it a clear age-related disease. In this review, we will focus on UV-induced carcinogenesis and photo aging along with natural protective mechanisms that reduce amount of “realized” solar radiation dose and UV-induced injury. -

Photophysics and Skin Penetration of Active Agents in a Commercial Sunscreen and Insect Repellent
PHOTOPHYSICS AND SKIN PENETRATION OF ACTIVE AGENTS IN A COMMERCIAL SUNSCREEN AND INSECT REPELLENT by DONALD PRETTYPAUL A Dissertation submitted to the Graduate School-Newark Rutgers, The State University of New Jersey In partial fulfillment of the requirements for the degree of Doctor of Philosophy Graduate Program in Chemistry written under the direction of Professor Richard Mendelsohn Professor Piotr Piotrowiak and approved by Newark, New Jersey October 2018 ©2018 Donald Prettypaul ALL RIGHTS RESERVED ABSTRACT OF DISSERTATION PHOTOPHYSICS AND SKIN PENETRATION OF ACTIVE AGENTS IN A COMMERCIAL SUNSCREEN AND INSECT REPELLENT By DONALD PRETTYPAUL Dissertation co-Directors: Professor Richard Mendelsohn Professor Piotr Piotrowiak This dissertation is focused on active agents in commercial sunscreen and insect repellent products. It consists of two parts, the first focusing on the photophysics of a sunscreen active agent and the second on the permeation and spatial distribution of the sunscreen active and an insect repellent active when these agents are applied to ex-vivo human skin. In the photochemistry study, ultrafast spectroscopy was used to study the excited state dynamics of the sunscreen molecule, Bemotrizinol. The work focused on the dissipation rates of the electronic excitation energy in different solvents. To complement the results from time-resolved femtosecond spectroscopy, Hartree- Fock UH/UHF 6-31G* calculations were used to characterize the ground and excited states potential energy surfaces. The results indicate that the excited state deactivation pathway follows a proton coupled electron transfer process which ii proceeds via a concerted mechanism. The dependencies on solvent polarity, viscosity, and H/D isotope effects, were investigated. Sunscreen products have been developed to protect skin from ultraviolet (UV) radiation; to achieve adequate protection, the sunscreen must be evenly applied and remain on the surface of the skin. -

New Technology Provides Cosmetically Elegant Photoprotection Zoe Diana Draelos, MD; Christian Oresajo; Margarita Yatskayer; Angelike Galdi; Isabelle Hansenne
COSMETIC CONSULTATION New Technology Provides Cosmetically Elegant Photoprotection Zoe Diana Draelos, MD; Christian Oresajo; Margarita Yatskayer; Angelike Galdi; Isabelle Hansenne he primary preventable cause of photoaging is United States, as there is no official rating system for exposure to UVA radiation. This wavelength UVA photoprotection yet. T emitted by the sun is present year round in all Ecamsule absorbs UVA radiation in the range of 320 to latitudes. Currently, the majority of sun-protective prod- 360 nm, but its peak absorbance occurs at 345 nm. It is ucts provide excellent UVB protection with minimal UVA typically combined with other organic sunscreen ingredi- protection. Although UVB protection is important in ents, such as avobenzone and octocrylene. Avobenzone, order to prevent sun damage to the skin from occurring, which is a photounstable photoprotectant, becomes UVA protection is equally important. New developments photostable when combined with octocrylene. These in raw material science have led to the manufacture of ingredients yield a photostable broad-spectrum sunscreen novel ingredients able to provide unprecedented photo- combination. However, the active sunscreen agents are protection COSin the UVA spectrum. DERMonly part of the formulation. Also important in sunscreen One of the most significant developments in cutaneous is the construction of the vehicle to deliver the photo- UVA protection was the discovery of ecamsule (Figure 1), protectants in an aesthetically pleasing manner, enticing also known as Mexoryl SX. Mexoryl SX, which has patients to wear the product. Sunscreens fail to be effec- the International Nomenclature of Cosmetic Ingredients tive if they remain in the bottle. name of terephthalylidene dicamphor sulfonic acid, is In order to evaluate the efficacy and tolerability of a water soluble. -

1. Background and Action Requested
June 27, 2019 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Comments Submitted to Docket No. 1978N-0018 (Formerly Docket No. FDA–1978–N– 0038) for “Sunscreen Drug Products for Over-the-Counter Human Use,” Regulatory Information No. 0910-AF43 DSM Nutritional Products LLC (“DSM”) is pleased to provide the following comments in response to the Food and Drug Administration’s (FDA’s) Tentative Final Monograph (“TFM”) for Sunscreen Drug Products Over-the-Counter (OTC) Human Use, published at 84 Fed. Reg. 6204 (February 26, 2019). As a global science-based company active in Nutrition, Health and Sustainable Living, DSM is a world leading supplier of vitamins, feed, food, pharmaceutical, cosmetic, personal care and aroma ingredients. In sunscreens, DSM is a leading world-wide provider of numerous UV filters, including Avobenzone. DSM takes human health and safety very seriously and believes that sunscreens are proven and effective interventions that help provide consumer protection against skin cancers and skin damage caused by the sun’s rays. DSM is committed to proactively supporting the safety and availability of existing and new sunscreen active ingredients that help protect public health. DSM requests that FDA modify its proposed rule taking into consideration the comments provided herein. 1. Background and Action Requested Skin cancer is a significant, and largely preventable, public health concern. Sunscreens have been widely and safely used by the general public for their photoprotective properties, including prevention of photocarcinogenesis and photoaging and management of photodermatoses for more than 40 years. FDA’s Sunscreen Monograph rule has identified the permitted active ingredients (UV-filters) for sunscreens since 1978. -

FDA Proposes Sunscreen Regulation Changes February 2019
FDA Proposes Sunscreen Regulation Changes February 2019 The U.S. Food and Drug Administration (FDA) regulates sunscreens to ensure they meet safety and eectiveness standards. To improve the quality, safety, and eectiveness of sunscreens, FDA issued a proposed rule that describes updated proposed requirements for sunscreens. Given the recognized public health benets of sunscreen use, Americans should continue to use broad spectrum sunscreen with SPF 15 or higher with other sun protective measures as this important rulemaking eort moves forward. Highlights of FDA’s Proposals Sunscreen active ingredient safety and eectiveness Two ingredients (zinc oxide and titanium dioxide) are proposed to be safe and eective for sunscreen use and two (aminobenzoic acid (PABA) and trolamine salicylate) are 1 proposed as not safe and eective for sunscreen use. FDA proposes that it needs more safety information for the remaining 12 sunscreen ingredients (cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, padimate O, sulisobenzone, oxybenzone, avobenzone). New proposed sun protection factor Sunscreen dosage forms (SPF) and broad spectrum Sunscreen sprays, oils, lotions, creams, gels, butters, pastes, ointments, and sticks are requirements 2 proposed as safe and eective. FDA 3 • Raise the maximum proposed labeled SPF proposes that it needs more data for from SPF 50+ to SPF 60+ sunscreen powders. • Require any sunscreen SPF 15 or higher to be broad spectrum • Require for all broad spectrum products SPF 15 and above, as SPF increases, broad spectrum protection increases New proposed label requirements • Include alphabetical listing of active ingredients on the front panel • Require sunscreens with SPF below 15 to include “See Skin Cancer/Skin Aging alert” on the front panel 4 • Require font and placement changes to ensure SPF, broad spectrum, and water resistance statements stand out Sunscreen-insect repellent combination 5 products proposed not safe and eective www.fda.gov. -

New Sunscreen Approval — Why So Complicated?
Watch Pages New Sunscreen Approval — Why So Complicated? Topically-applied sunscreen product use is a billion-dollar These popular sunscreens have been submitted to the industry. The use of these products over the last 40 US Food and Drug Administration (FDA) over the last 15 years has changed dramatically from a “niche” market of years for approval. The FDA has approved none of these fair-skinned individuals who used small amounts on an products — which are regulated as drugs in the US — irregular basis to the current market of a large swath of despite years of use in Europe, Asia, or Latin America, the adult and paediatric population, fair-skinned or not, where they are regulated as cosmetics. The last over-the- over larger areas of their bodies on a much more regular counter (OTC) sunscreen approved by the FDA was in the basis — in some cases, daily. late 1990s. That said, the agency has not rejected these products either. Benefits of sunscreen use (SPF 15+) are widely accepted: sunburn prevention, skin-aging reduction, and, Instead, the products are in a nebulous regulatory of particular importance, skin cancer risk reduction. The area due to their classification and lack of criteria to benefits and increased usage have resulted in demand determine the type of clinical data needed to prove they for and development of a wide array of new sunscreen are “generally recognised as safe and effective” (GRASE), products over the last 20 years. These modern products an essential designation needed for OTC approval. This offer enhanced protection against the ultraviolet A (UVA) designation is usually granted, and the drug thereby rays that play a larger part in skin cancer than was once regulated, under what is called the OTC drug monograph believed, as well as the traditional protection against process. -

WO 2013/036901 A2 14 March 2013 (14.03.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/036901 A2 14 March 2013 (14.03.2013) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 8/30 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/US2012/054376 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 10 September 2012 (10.09.2012) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (26) Publication Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, (30) Priority Data: ZM, ZW. 61/532,701 9 September 201 1 (09.09.201 1) US (84) Designated States (unless otherwise indicated, for every (71) Applicant (for all designated States except US): UNIVER¬ kind of regional protection available): ARIPO (BW, GH, SITY OF FLORIDA RESEARCH FOUNDATION, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, INC.