FDA Proposes Sunscreen Regulation Changes February 2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Report of the Advisory Group to Recommend Priorities for the IARC Monographs During 2020–2024
IARC Monographs on the Identification of Carcinogenic Hazards to Humans Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 CONTENTS Introduction ................................................................................................................................... 1 Acetaldehyde (CAS No. 75-07-0) ................................................................................................. 3 Acrolein (CAS No. 107-02-8) ....................................................................................................... 4 Acrylamide (CAS No. 79-06-1) .................................................................................................... 5 Acrylonitrile (CAS No. 107-13-1) ................................................................................................ 6 Aflatoxins (CAS No. 1402-68-2) .................................................................................................. 8 Air pollutants and underlying mechanisms for breast cancer ....................................................... 9 Airborne gram-negative bacterial endotoxins ............................................................................. 10 Alachlor (chloroacetanilide herbicide) (CAS No. 15972-60-8) .................................................. 10 Aluminium (CAS No. 7429-90-5) .............................................................................................. 11 -

Safety of Oxybenzone: Putting Numbers Table
Safety of Oxybenzone: Putting Numbers Table. Years of Daily Sunscreen Application Required by an Average US Woman to Reach Systemic Levels Into Perspective of Oxybenzone per Unit of Body Mass Equivalent to Those Given to Immature Rats10 xybenzone, an organic UV-B and short-wave UV-A filter, has been available for over 40 years1; Scenario Scenario Scenario it is widely used in sunscreens and other con- Characteristic 1 2 3 O 2 sumer products in the United States. The Centers for Dis- Body surface area covered, % 100 100 25 ease Control and Prevention has estimated the preva- Application dose, mg/cm2/d211 lence of oxybenzone exposure in the general US Application amount required, 30.00 15.00 3.75 population to be 96.8%.3 In the past few years, oxyben- mL/d Time required, y 34.6 69.3 277.0 zone has received increasing attention as a potentially harmful compound. Initial concerns arose when a re- port demonstrated systemic absorption of oxybenzone in humans at a rate of 1% to 2% after topical applica- For the purposes of this estimation, a generous in-use dose of 1 mg/cm2 was used,13-16 and it was assumed that cov- tion.4 Similar or higher rates of cutaneous absorption in 5-9 erage of 25% of the human BSA was limited to the face, human subjects have been observed. The potential for neck, hands, and arms.17 biological effects, however, were first published in a study 10 by Schlumpf et al demonstrating uterotropic effects in Results. The numbers of years of daily application re- immature rats after oral administration of oxybenzone; quired to obtain the equivalent amount of sunscreen un- it should be noted that the estrogenic effect detected was der the 3 scenarios are listed in the Table. -

May 23, 2007 Office of Pesticide Programs
May 23, 2007 Office of Pesticide Programs (OPP) Regulatory Public Docket (7502P) Environmental Protection Agency 1200 Pennsylvania Ave., NW Washington, DC 20460-0001 RE: Insect Repellent-Sunscreen Combination Products [EPA-HQ-OPP-2007-0087] Beyond Pesticides appreciates the prudent contemplation of insect repellent-sunscreen combination products EPA proposed in the reregistration eligibility decision (RED) for N,N-diethyl-meta-toluamide (DEET). We also appreciate this opportunity to share our concerns over these products. Beyond Pesticides interest in this issue lies in our effort to restrict pesticide use in a manner that protects public health and the environment, and to advance alternatives that eliminate dependency on toxic chemicals. We oppose the reregistration of all DEET-sunscreen combination products for the following reasons: 1. DEET exposure can result in negative health effects. As the agency notes, the registration of DEET is unusual in that it is one of few residential-use pesticides that is applied directly to the skin. The result is that the public is being exposed to a pesticide that has the ability to cause in lab animals increased fetal loss, bone and skeleton abnormalities in the offspring of rabbits, birth defects in birds, reduction in size of the testes and degeneration, and has produced abnormal sperm with reduced motility. Additionally, the public is directly applying a chemical to their skin that is demonstrated to cross the placenta and move into fetal blood in humans, has the ability to cause mutagenicity and oxidative stress, can decrease sensory and motor skills, causes skin irritation and kills brain cells.1 2. Sunscreen exposure can result in negative health effects. -

A Thesis Entitled Evaluating UVB and UVA Boosting Technologies For
A Thesis entitled Evaluating UVB and UVA Boosting Technologies for Chemical and Physical Sunscreens by An Ngoc Hiep Huynh Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science Degree in Pharmaceutical Sciences Industrial Pharmacy ___________________________________________ Gabriella Baki, Ph.D., Committee Chair ___________________________________________ Jerry Nesamony, Ph.D., Committee Member ___________________________________________ Matthew W. Liberatore, Ph.D., Committee Member ___________________________________________ Dr. Amanda C. Bryant-Friedrich, Dean College of Graduate Studies The University of Toledo May 2020 Copyright 2020 An Ngoc Hiep Huynh This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Evaluating UVB and UVA Boosting Technologies for Chemical and Physical Sunscreens by An Ngoc Hiep Huynh Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science Degree in Pharmaceutical Sciences Industrial Pharmacy The University of Toledo May 2020 There are currently 14 organic and 2 inorganic UV filters approved in the United States. Due to coral reef safety concerns, octinoxate and oxybenzone have been banned in Hawaii, Key West, FL and the US Virgin Islands; and octocrylene is also being studied for its potential impact on coral reef safety, leaving 11 organic UV filters as viable options for sunscreen manufacturers – with limitations on their combination. Since consumers are always looking for sunscreens with high SPF and broad-spectrum protection, the need for UVB and UVA protection boosting technologies is greater than ever. In a preliminary study, about two dozen emollients were scanned for their SPF boosting capability with selected organic UV filters. -

GAO-18-61, SUNSCREEN: FDA Reviewed Applications For
United States Government Accountability Office Report to Congressional Committees November 2017 SUNSCREEN FDA Reviewed Applications for Additional Active Ingredients and Determined More Data Needed GAO-18-61 November 2017 SUNSCREEN FDA Reviewed Applications for Additional Active Ingredients and Determined More Data Needed Highlights of GAO-18-61, a report to congressional committees Why GAO Did This Study What GAO Found Using sunscreen as directed with other The Food and Drug Administration (FDA), within the Department of Health and sun protective measures may help Human Services, implemented requirements for reviewing applications for reduce the risk of skin cancer—the sunscreen active ingredients within time frames set by the Sunscreen Innovation most common form of cancer in the Act, which was enacted in November 2014. For example, the agency issued a United States. In the United States, guidance document on safety and effectiveness testing in November 2016. sunscreen is considered an over-the- counter drug, which is a drug available As of August 2017, all applications for sunscreen active ingredients remain to consumers without a prescription. pending after the agency determined more safety and effectiveness data are Some sunscreen active ingredients not needed. By February 2015, FDA completed its initial review of the safety and currently marketed in the United States effectiveness data for each of the eight pending applications, as required by the have been available in products in act. FDA concluded that additional data are needed to determine that the other countries for more than a ingredients are generally recognized as safe and effective (GRASE), which is decade. Companies that manufacture needed so that products using the ingredients can subsequently be marketed in some of these ingredients have sought the United States without FDA’s premarket approval. -

Toxic Effects
Chapter 8 Toxic effects Toxic and other adverse effects studies of tens or hundreds of patients spectrum to which the skin is exposed of sunscreens (Thune, 1984; English et al., 1987; (Gasparro et al., 1998). Since UVB is the In order for a sunscreen to have a toxic Lenique et al., 1992; Szczurko et al., primary stimulus for adaptation of the effect on living tissues, it must penetrate 1994; Trevisi et al., 1994; Gonçalo et al., skin to sunlight, less adaptation might be the skin. There is some evidence that 1995; Ang et al., 1998) and reviews expected to develop in individuals who this can occur (see p. 63 et seq.). (Dromgoole & Maibach, 1990; Gonzalez use sunscreens regularly. The adaptive & Gonzalez, 1996; Schauder & Ippen, responses include thickening of the epi- Human studies 1997). In the past, PABA and its esters dermis and transfer of melanin-contain- No published studies of toxic effects in were the most commonly reported ing granules to keratinocytes (tanning) humans were available to the Working contact and photoallergens in sun- (Fig. 44), which reduces the trans- Group. screens (Funk et ai., 1997), and this find- parency of the skin to UVA and UVB ing contributed to a reduction in their use (Fusaro et al., 1966; Olson et al., 1973). Contact sensitivity in sunscreens. The contact or photocon- Several reports showed that UVR- There are numerous reports of cases of tact allergen in sunscreens most induced injury, such as dermal connec- allergic reactions and photoreactivity to frequently cited today is benzophenone- tive tissue damage and sunburn cell for- sunscreens, but the prevalence of this 3, followed by dibenzoyl methanes. -

Octinoxate, Octisalate, Avobenzone, Ensulizole, Homosalate
TONYMOLY MAGIC FOOD MANGO MILD SUN BLOCK- octinoxate, octisalate, avobenzone, ensulizole, homosalate cream TONYMOLY CO.,LTD Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies. ---------- ACTIVE INGREDIENT Active ingredients: Ethylhexyl Methoxycinnamate 6.9%, Ethylhexyl Salicylate 4.5%, Butyl Methoxydibenzoylmethane 3.5%, Phenylbenzimidazole Sulfonic Acid 3.5%, Homosalate 3.0% INACTIVE INGREDIENT Inactive ingredients: Water, Butylene Glycol, Alcohol Denat., Octocrylene, Phenethyl Benzoate, Aminomethyl Propanol, Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine, Triceteareth-4 Phosphate, Glycol Stearate, Ammonium Acryloyldimethyltaurate/VP Copolymer, Carbomer, PEG-2 Stearate, Fragrance(Parfum), Phenoxyethanol, Glyceryl Caprylate, Caprylyl Glycol, Mangifera Indica (Mango) Seed Butter, Disodium EDTA, Citrus Limon (Lemon) Fruit Extract, Musa Sapientum (Banana) Fruit Extract, Propylene Glycol, 1,2-Hexanediol, Citrus Aurantium Dulcis (Orange) Fruit Extract, Mangifera Indica (Mango) Fruit Extract, Psidium Guajava Fruit Extract, Citrus Paradisi (Grapefruit) Fruit Extract, Cocos Nucifera (Coconut) Fruit Extract, Actinidia Chinensis (Kiwi) Fruit Extract, Carica Papaya (Papaya) Fruit Extract, Ethylhexylglycerin PURPOSE Purpose: Sunscreen WARNINGS Warnings: For external use only Do not use on damaged or broken skin Stop use and ask a doctor if irritation occurs Keep out of reach of children DESCRIPTION Uses: - helps prevent sunburn - If used as directed with other sun protection measures Directions: decreases the risk of skin cancer and early skin aging caused by the sun Directions: For sunscreen use: - apply liberally 15 minutes before sun exposure - use a water resistant sunscreen if swimming or sweating - reapply at least every 2 hours - Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. -
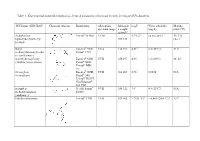
Table 1 - Experimental and Predicted Physical-Chemical Parameters of the Most Recently Investigated UV-Absorbers
Table 1 - Experimental and predicted physical-chemical parameters of the most recently investigated UV-absorbers. INCI name (INN/XAN) Chemical structure Brand name Absorption Molecula LogP Water solubility Melting spectrum range r weight (mg/L) point (°C) (g/mol)4 diethylamino Uvinul® A Plus UVA1 5.7-6.21 <0.01 (20°C) 1 54; 314 hydroxybenzoyl hexyl 397.515 (dec.) 1 benzoate Butyl Eusolex® 9020, UVA 310.393 4.514 2.2 (25°C)4 83.54 methoxydibenzoylmetha Parsol® 1789 ne (avobenzone) 4-methylbenzylidene Eusolex® 6300 UVB 258.397 4.95 1.3 (20°C) 66–68 camphor (enzacamene) Parsol® 5000 Uvinul® MBC 95 Octocrylene Eusolex® OCR, UVB 361.485 6.783 0.00383 N/A (octocrilene) Parsol® 340, Uvinul® N539T, NeoHeliopan® 303 USP isoamyl p- Neo Heliopan® UVB 248.322 3.61 4.9 (25°C)1 N/A methoxycinnamate E1000 (amiloxate) Ethylhexyl triazone Uvinul® T150 UVB 823.092 > 7(20 °C) 6 < 0.001 (20.0 °C) 6 1296 Ethylhexyl Parsol® MCX, UVB 290.403 6.14 0.041 (24 °C and N/A methoxycinnamate Heliopan® New pH 7.1) 4 (octinoxate) Ethylhexyl dimethyl Escalol™ 507 UVB 277.4084 5.774 0.54 (25 °C) 4 N/A PABA (padimate-O) Arlatone 507 Eusolex 6007 benzophenone-3 Eusolex® 4360 UVA2+ UVB 228.247 3.72 3.7 (20°C) 2 62-652 (oxybenzone) bis-ethylhexyloxyphenol Tinosorb® S UVA1+UVB 627.826 12.61 <10-4 80.401 methoxyphenol triazine (bemotrizinol) Phenylbenzimidazole Eusolex® 232 UVA2+ UVB 274.2945 -1.1 (pH 5) > 30% (As sodium N/A sulfonic acid Parsol® HS -2.1 (pH 8)5 or (ensulizole) Neo Heliopan® triethanolammoniu Hydro m salt at 20°C) 5 1 (3) 2 (34) 3 (44) 4 Pubchem 5 SCCP/1056/06 Opinion on phenylbenzimidazole sulfonic acid and its salts 6 BASF safety data sheet Table 2 – In vitro studies for the assessment of skin permeation/penetration of sunscreens. -

Sun Protection, Sunscreens and Vitamin D
SunSun protection,protection, sunscreenssunscreens andand VitaminVitamin DD GPGP NationalNational ConferenceConference RotoruaRotorua EnergyEnergy EventsEvents CentreCentre JuneJune 20092009 Dr. Louise Reiche Dermatologist New Zealand Dermatological Society Incorporated MelanomaMelanoma SkinSkin cancercancer andand sunlightsunlight Exposure to UVR causes > 90% of skin cancers Skin cancer is commonest cancer in NZ >50,000 new cases per year ~300 deaths per year ~$33.4 NZ million per year International Agency for Research on Cancer. IARC Monographs on the evaluation of carcinogenic risks to humans. Solar ultraviolet radiation. Lyon: International Agency for Research on Cancer, 1992. Armstrong BK. How sun exposure causes skin cancer. In: Hill D, Elwood JM, English DR, Eds. Prevention of Skin Cancer. Dordrecht: Kluwer Academic Publishers, 2004. O’Dea D. The Costs of Skin Cancer to New Zealand. Wellington: Cancer Society of New Zealand, 2000. New Zealand Health Information Service. Cancer, New Registrations and Deaths. Wellington: New Zealand Health Information Service, 2004. MelanomaMelanoma 1842 new cases in 2002 328 directly attributable to severe sunburn (Sneyd and Cox 2006) Authors recommended, “to reduce burden of melanoma in NZ, need to prevent excessive sun exposure and (facilitate) early diagnosis” Whilst cancer overall is rare in adolescence, melanoma was commonest cancer MelanomaMelanoma NZ incidence and death rate among world highest 56.2/100,000 in European population of Auckland highest reported worldwide men -

Two-Step Semi-Microscale Preparation of a Cinnamate Ester Sunscreen Analog W
In the Laboratory edited by The Microscale Laboratory R. David Crouch Dickinson College Carlisle, PA 17013-2896 Two-Step Semi-Microscale Preparation of a Cinnamate Ester Sunscreen Analog W Ryan G. Stabile and Andrew P. Dicks* Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario, Canada, M5S 3H6; *[email protected] Several laboratory experiments concerning the physical A two-step procedure using 4-methoxybenzaldehyde as properties of sunscreens have appeared in this Journal dur- the starting material (Scheme I) synthesizes ethyl trans-4- ing the last decade (1–5). These include quantification of methoxycinnamate 1. In the first laboratory session students commercial formulations by liquid chromatography (1) and are exposed to an important carbon–carbon bond forming ultraviolet (UV) spectrophotometry (2–4). The photochem- condensation reaction. The Verley–Doebner modification of istry of sunscreens has also been reviewed (6). Significantly, the Knoevenagel condensation (11) affords facile synthesis a student procedure focusing on multistep sunscreen synthesis of trans-4-methoxycinnamic acid, which is isolated and char- and spectroscopic analysis has not, to our knowledge, been acterized. During the second period, esterification is effected reported. Given the current high profile nature of skin can- by a cesium base mediated O-alkylation approach. This ex- cer (7) and media attention towards sunscreens, we designed emplifies the so-called “cesium effect” (12) and the useful- a two-step synthetic pathway towards an analog of a com- ness of cesium carboxylates as nucleophiles in SN2 reactions. mercially available UV light blocker. This methodology is in- In addition to stimulating class enthusiasm towards syn- corporated into a third-year undergraduate organic synthesis thetic chemistry, this experiment impresses many organic course at the University of Toronto. -

New Technology Provides Cosmetically Elegant Photoprotection Zoe Diana Draelos, MD; Christian Oresajo; Margarita Yatskayer; Angelike Galdi; Isabelle Hansenne
COSMETIC CONSULTATION New Technology Provides Cosmetically Elegant Photoprotection Zoe Diana Draelos, MD; Christian Oresajo; Margarita Yatskayer; Angelike Galdi; Isabelle Hansenne he primary preventable cause of photoaging is United States, as there is no official rating system for exposure to UVA radiation. This wavelength UVA photoprotection yet. T emitted by the sun is present year round in all Ecamsule absorbs UVA radiation in the range of 320 to latitudes. Currently, the majority of sun-protective prod- 360 nm, but its peak absorbance occurs at 345 nm. It is ucts provide excellent UVB protection with minimal UVA typically combined with other organic sunscreen ingredi- protection. Although UVB protection is important in ents, such as avobenzone and octocrylene. Avobenzone, order to prevent sun damage to the skin from occurring, which is a photounstable photoprotectant, becomes UVA protection is equally important. New developments photostable when combined with octocrylene. These in raw material science have led to the manufacture of ingredients yield a photostable broad-spectrum sunscreen novel ingredients able to provide unprecedented photo- combination. However, the active sunscreen agents are protection COSin the UVA spectrum. DERMonly part of the formulation. Also important in sunscreen One of the most significant developments in cutaneous is the construction of the vehicle to deliver the photo- UVA protection was the discovery of ecamsule (Figure 1), protectants in an aesthetically pleasing manner, enticing also known as Mexoryl SX. Mexoryl SX, which has patients to wear the product. Sunscreens fail to be effec- the International Nomenclature of Cosmetic Ingredients tive if they remain in the bottle. name of terephthalylidene dicamphor sulfonic acid, is In order to evaluate the efficacy and tolerability of a water soluble. -

1. Background and Action Requested
June 27, 2019 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Comments Submitted to Docket No. 1978N-0018 (Formerly Docket No. FDA–1978–N– 0038) for “Sunscreen Drug Products for Over-the-Counter Human Use,” Regulatory Information No. 0910-AF43 DSM Nutritional Products LLC (“DSM”) is pleased to provide the following comments in response to the Food and Drug Administration’s (FDA’s) Tentative Final Monograph (“TFM”) for Sunscreen Drug Products Over-the-Counter (OTC) Human Use, published at 84 Fed. Reg. 6204 (February 26, 2019). As a global science-based company active in Nutrition, Health and Sustainable Living, DSM is a world leading supplier of vitamins, feed, food, pharmaceutical, cosmetic, personal care and aroma ingredients. In sunscreens, DSM is a leading world-wide provider of numerous UV filters, including Avobenzone. DSM takes human health and safety very seriously and believes that sunscreens are proven and effective interventions that help provide consumer protection against skin cancers and skin damage caused by the sun’s rays. DSM is committed to proactively supporting the safety and availability of existing and new sunscreen active ingredients that help protect public health. DSM requests that FDA modify its proposed rule taking into consideration the comments provided herein. 1. Background and Action Requested Skin cancer is a significant, and largely preventable, public health concern. Sunscreens have been widely and safely used by the general public for their photoprotective properties, including prevention of photocarcinogenesis and photoaging and management of photodermatoses for more than 40 years. FDA’s Sunscreen Monograph rule has identified the permitted active ingredients (UV-filters) for sunscreens since 1978.