Bupha T 2018 Moutier La
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Thesis Entitled Evaluating UVB and UVA Boosting Technologies For
A Thesis entitled Evaluating UVB and UVA Boosting Technologies for Chemical and Physical Sunscreens by An Ngoc Hiep Huynh Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science Degree in Pharmaceutical Sciences Industrial Pharmacy ___________________________________________ Gabriella Baki, Ph.D., Committee Chair ___________________________________________ Jerry Nesamony, Ph.D., Committee Member ___________________________________________ Matthew W. Liberatore, Ph.D., Committee Member ___________________________________________ Dr. Amanda C. Bryant-Friedrich, Dean College of Graduate Studies The University of Toledo May 2020 Copyright 2020 An Ngoc Hiep Huynh This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Evaluating UVB and UVA Boosting Technologies for Chemical and Physical Sunscreens by An Ngoc Hiep Huynh Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science Degree in Pharmaceutical Sciences Industrial Pharmacy The University of Toledo May 2020 There are currently 14 organic and 2 inorganic UV filters approved in the United States. Due to coral reef safety concerns, octinoxate and oxybenzone have been banned in Hawaii, Key West, FL and the US Virgin Islands; and octocrylene is also being studied for its potential impact on coral reef safety, leaving 11 organic UV filters as viable options for sunscreen manufacturers – with limitations on their combination. Since consumers are always looking for sunscreens with high SPF and broad-spectrum protection, the need for UVB and UVA protection boosting technologies is greater than ever. In a preliminary study, about two dozen emollients were scanned for their SPF boosting capability with selected organic UV filters. -

Octinoxate, Octisalate, Avobenzone, Ensulizole, Homosalate
TONYMOLY MAGIC FOOD MANGO MILD SUN BLOCK- octinoxate, octisalate, avobenzone, ensulizole, homosalate cream TONYMOLY CO.,LTD Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies. ---------- ACTIVE INGREDIENT Active ingredients: Ethylhexyl Methoxycinnamate 6.9%, Ethylhexyl Salicylate 4.5%, Butyl Methoxydibenzoylmethane 3.5%, Phenylbenzimidazole Sulfonic Acid 3.5%, Homosalate 3.0% INACTIVE INGREDIENT Inactive ingredients: Water, Butylene Glycol, Alcohol Denat., Octocrylene, Phenethyl Benzoate, Aminomethyl Propanol, Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine, Triceteareth-4 Phosphate, Glycol Stearate, Ammonium Acryloyldimethyltaurate/VP Copolymer, Carbomer, PEG-2 Stearate, Fragrance(Parfum), Phenoxyethanol, Glyceryl Caprylate, Caprylyl Glycol, Mangifera Indica (Mango) Seed Butter, Disodium EDTA, Citrus Limon (Lemon) Fruit Extract, Musa Sapientum (Banana) Fruit Extract, Propylene Glycol, 1,2-Hexanediol, Citrus Aurantium Dulcis (Orange) Fruit Extract, Mangifera Indica (Mango) Fruit Extract, Psidium Guajava Fruit Extract, Citrus Paradisi (Grapefruit) Fruit Extract, Cocos Nucifera (Coconut) Fruit Extract, Actinidia Chinensis (Kiwi) Fruit Extract, Carica Papaya (Papaya) Fruit Extract, Ethylhexylglycerin PURPOSE Purpose: Sunscreen WARNINGS Warnings: For external use only Do not use on damaged or broken skin Stop use and ask a doctor if irritation occurs Keep out of reach of children DESCRIPTION Uses: - helps prevent sunburn - If used as directed with other sun protection measures Directions: decreases the risk of skin cancer and early skin aging caused by the sun Directions: For sunscreen use: - apply liberally 15 minutes before sun exposure - use a water resistant sunscreen if swimming or sweating - reapply at least every 2 hours - Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. -
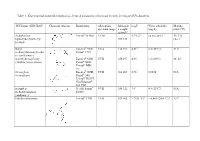
Table 1 - Experimental and Predicted Physical-Chemical Parameters of the Most Recently Investigated UV-Absorbers
Table 1 - Experimental and predicted physical-chemical parameters of the most recently investigated UV-absorbers. INCI name (INN/XAN) Chemical structure Brand name Absorption Molecula LogP Water solubility Melting spectrum range r weight (mg/L) point (°C) (g/mol)4 diethylamino Uvinul® A Plus UVA1 5.7-6.21 <0.01 (20°C) 1 54; 314 hydroxybenzoyl hexyl 397.515 (dec.) 1 benzoate Butyl Eusolex® 9020, UVA 310.393 4.514 2.2 (25°C)4 83.54 methoxydibenzoylmetha Parsol® 1789 ne (avobenzone) 4-methylbenzylidene Eusolex® 6300 UVB 258.397 4.95 1.3 (20°C) 66–68 camphor (enzacamene) Parsol® 5000 Uvinul® MBC 95 Octocrylene Eusolex® OCR, UVB 361.485 6.783 0.00383 N/A (octocrilene) Parsol® 340, Uvinul® N539T, NeoHeliopan® 303 USP isoamyl p- Neo Heliopan® UVB 248.322 3.61 4.9 (25°C)1 N/A methoxycinnamate E1000 (amiloxate) Ethylhexyl triazone Uvinul® T150 UVB 823.092 > 7(20 °C) 6 < 0.001 (20.0 °C) 6 1296 Ethylhexyl Parsol® MCX, UVB 290.403 6.14 0.041 (24 °C and N/A methoxycinnamate Heliopan® New pH 7.1) 4 (octinoxate) Ethylhexyl dimethyl Escalol™ 507 UVB 277.4084 5.774 0.54 (25 °C) 4 N/A PABA (padimate-O) Arlatone 507 Eusolex 6007 benzophenone-3 Eusolex® 4360 UVA2+ UVB 228.247 3.72 3.7 (20°C) 2 62-652 (oxybenzone) bis-ethylhexyloxyphenol Tinosorb® S UVA1+UVB 627.826 12.61 <10-4 80.401 methoxyphenol triazine (bemotrizinol) Phenylbenzimidazole Eusolex® 232 UVA2+ UVB 274.2945 -1.1 (pH 5) > 30% (As sodium N/A sulfonic acid Parsol® HS -2.1 (pH 8)5 or (ensulizole) Neo Heliopan® triethanolammoniu Hydro m salt at 20°C) 5 1 (3) 2 (34) 3 (44) 4 Pubchem 5 SCCP/1056/06 Opinion on phenylbenzimidazole sulfonic acid and its salts 6 BASF safety data sheet Table 2 – In vitro studies for the assessment of skin permeation/penetration of sunscreens. -

FDA Proposes Sunscreen Regulation Changes February 2019
FDA Proposes Sunscreen Regulation Changes February 2019 The U.S. Food and Drug Administration (FDA) regulates sunscreens to ensure they meet safety and eectiveness standards. To improve the quality, safety, and eectiveness of sunscreens, FDA issued a proposed rule that describes updated proposed requirements for sunscreens. Given the recognized public health benets of sunscreen use, Americans should continue to use broad spectrum sunscreen with SPF 15 or higher with other sun protective measures as this important rulemaking eort moves forward. Highlights of FDA’s Proposals Sunscreen active ingredient safety and eectiveness Two ingredients (zinc oxide and titanium dioxide) are proposed to be safe and eective for sunscreen use and two (aminobenzoic acid (PABA) and trolamine salicylate) are 1 proposed as not safe and eective for sunscreen use. FDA proposes that it needs more safety information for the remaining 12 sunscreen ingredients (cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, padimate O, sulisobenzone, oxybenzone, avobenzone). New proposed sun protection factor Sunscreen dosage forms (SPF) and broad spectrum Sunscreen sprays, oils, lotions, creams, gels, butters, pastes, ointments, and sticks are requirements 2 proposed as safe and eective. FDA 3 • Raise the maximum proposed labeled SPF proposes that it needs more data for from SPF 50+ to SPF 60+ sunscreen powders. • Require any sunscreen SPF 15 or higher to be broad spectrum • Require for all broad spectrum products SPF 15 and above, as SPF increases, broad spectrum protection increases New proposed label requirements • Include alphabetical listing of active ingredients on the front panel • Require sunscreens with SPF below 15 to include “See Skin Cancer/Skin Aging alert” on the front panel 4 • Require font and placement changes to ensure SPF, broad spectrum, and water resistance statements stand out Sunscreen-insect repellent combination 5 products proposed not safe and eective www.fda.gov. -

Les Filtres UV Dans Les Cosmétiques : Une Présence Obligatoire ?
UNIVERSITÉ DE NANTES UFR SCIENCES PHARMACEUTIQUES ET BIOLOGIQUES ____________________________________________________________________________ ANNÉE 2015 N° THÈSE pour le DIPLÔME D’ÉTAT DE DOCTEUR EN PHARMACIE par Anouk POCHAT Présentée et soutenue publiquement le 14 décembre 2015 Les filtres UV dans les cosmétiques : une présence obligatoire ? Président : Mme Laurence Coiffard, Professeur des universités, Laboratoire de Pharmacie industrielle et Cosmétologie Membres du jury : Directeur de thèse : Mme Céline Couteau, Maître de conférences, HDR, Laboratoire de Pharmacie industrielle et Cosmétologie Mme Françoise PEIGNE , Maitre de conférences à la retraite Page 1 Remerciements A mon président de jury, Professeur à la faculté des sciences pharmaceutiques de Nantes J’exprime mes profonds remerciements à Mme Coiffard, pour m’avoir fait l’honneur de présider mon jury de thèse. A mon directeur de thèse, Maître de conférences à la faculté de Pharmacie de Nantes Je remercie Mme Couteau pour m’avoir conseillée et guidée tout au long de mon travail. A Madame Françoise PEIGNE, Docteur en Pharmacie, Je remercie Mme Peigné d’avoir accepté d’assister à ma soutenance. A ma mère, Je te remercie de m’avoir soutenue et encouragée tout au long de mes études. A mon conjoint, Je te remercie pour ta patience, ton écoute et ton soutien. A mes frères, Je vous remercie pour vos encouragements Page 2 I.Introduction Une exposition prolongée aux UVA et aux UVB peut avoir de graves conséquences sur la santé comme, par exemple, la survenue de cancers cutanés (Aubin F., 2001). Les filtres UV permettent d’assurer une protection dans les domaines UVA et/ou UVB. On en trouve dans les produits de protection solaire que le public utilise ponctuellement lors des expositions prolongées au soleil. -

Annex VI, Last Update: 02/08/2021
File creation date: 03/10/2021 Annex VI, Last update: 22/09/2021 LIST OF UV FILTERS ALLOWED IN COSMETIC PRODUCTS Substance identification Conditions Wording of Reference Maximum conditions of Product Type, concentration Update date number Chemical name / INN / XAN Name of Common Ingredients Glossary CAS Number EC Number Other use and body parts in ready for use warnings preparation 2 N,N,N-Trimethyl-4-(2-oxoborn-3-ylidenemethyl CAMPHOR BENZALKONIUM 52793-97-2 258-190-8 6% 15/10/2010 ) anilinium methyl sulphate METHOSULFATE 3 Benzoic acid, 2-hydroxy-, HOMOSALATE 118-56-9 204-260-8 10% 02/08/2021 3,3,5-trimethylcyclohexyl ester / Homosalate 4 2-Hydroxy-4-methoxybenzophenone / BENZOPHENONE-3 131-57-7 205-031-5 6% Reg (EU) Not more than Contains 02/08/2021 Oxybenzone 2017/238 of 10 0,5 % to protect Benzophenone-3 February 2017- product (1) date of formulation application from September 2017 6 2-Phenylbenzimidazole-5-sulphonic acid and its PHENYLBENZIMIDAZOLE SULFONIC 27503-81-7 248-502-0 8%(as acid) 08/03/2011 potassium, sodium and triethanolamine salts / ACID Ensulizole 7 3,3'-(1,4-Phenylenedimethylene) bis TEREPHTHALYLIDENE DICAMPHOR 92761-26-7 / 410-960-6 / - 10%(as acid) 26/10/2010 (7,7-dimethyl-2-oxobicyclo-[2.2.1] SULFONIC ACID 90457-82-2 hept-1-ylmethanesulfonic acid) and its salts / Ecamsule 8 1-(4-tert-Butylphenyl)-3-(4-methoxyphenyl) BUTYL 70356-09-1 274-581-6 5% 15/10/2010 propane-1,3-dione / Avobenzone METHOXYDIBENZOYLMETHANE 9 alpha-(2-Oxoborn-3-ylidene)toluene-4-sulphoni BENZYLIDENE CAMPHOR SULFONIC 56039-58-8 - 6%(as acid) -

EWG Petitions CDC to Conduct Biomonitoring Studies for Common Sunscreen Chemicals
EWG Petitions CDC To Conduct Biomonitoring Studies for Common Sunscreen Chemicals May 22, 2019 To: U.S. Department of Health and Human Services Centers for Disease Control and Prevention National Center for Environmental Health Agency for Toxic Substances and Disease Registry 4770 Buford Hwy, NE Atlanta, GA 30341 Patrick Breysse, Ph.D., CIH Environmental Working Group (EWG), a nonprofit research and advocacy organization with headquarters in Washington, D.C., is petitioning the Centers for Disease Control and Prevention to add common sunscreen chemicals to the CDC’s Biomonitoring Program. EWG has been doing research on sunscreen ingredients since 2007, helping to educate the public about the importance of using sunscreens for health protection, as well as providing information about health risks that may be associated with certain ingredients used in sunscreen products. In response to a significant increase in the use of sunscreens in the United States and the associated increased potential for systemic exposure to the ingredients in these products, in February 2019, the Food and Drug Administration proposed a new rule for sunscreen products.1 The proposed rule would require sunscreen active ingredients to be assessed for their propensity to absorb through the skin and overall safety. Recently, the FDA completed tests on the absorbance of four common sunscreen active ingredients: avobenzone, oxybenzone, octocrylene, and ecamsule. As reported in a study published by the Journal of American Medical Association in May 2019,2 application of all four tested sunscreen ingredients resulted in plasma concentrations that exceeded the 0.5 ng/mL threshold proposed by the FDA for waiving systemic carcinogenicity studies as well as developmental and reproductive toxicity studies. -

Sun Protection
DRUG NEWS Recommending the Best Sun Protection Clinical Pearls: o Recommend a “broad-spectrum” sunscreen – one that covers UVB, UVA1, and UVA2. o Recommend SPF 30-50 o Advise on non-pharmacological sun protection methods o Emphasize proper sunscreen application technique o Emphasize skin protection when taking drugs known to cause photosensitivity. Familiarize yourself with known implicated drugs by referring to appendix 2. Background 1-4 The sun emits 3 types of ultraviolet (UV) radiation: UVC (100-290 nm), UVB (290-320 nm), and UVA (320 -400 nm). UVA rays can be further divided into the shorter UVA2 rays and the longer UVA1 rays. UVC rays, the shortest rays, are completely absorbed by the ozone layer, whereas UVB rays penetrate the epidermis and UVA rays, the longest rays, penetrate into the dermis. The main consequence of UVB irradiation is sunburn, but can also include immunosuppression and skin cancer. Consequences of UVA radiation include: phototoxicity (i.e. involvement in drug-induced sun sensitivity reactions), photo-aging, immunosuppression, and skin cancer. What is SPF? 2,5,6,7 It is easy to be misled by Sun Protection Factors (SPF). SPF is assessed through a standardized test by finding the ratio of the minimal dose of solar radiation that produces perceptible erythema (i.e., minimal erythema dose) on sunscreen-protected skin compared with unprotected skin. Sunburn is caused primarily by UVB rays (and shorter UVA2 rays), and thus SPF indicates mostly UVB protection. However, UVA protection is equally important since it is responsible for photo-aging and cancer. Therefore, it is important to look for the phrase “broad spectrum” when choosing a sunscreen as broad spectrum indicates both UVB and UVA protection. -

Food and Drug Administration, HHS § 352.20
Food and Drug Administration, HHS § 352.20 (c) Cinoxate up to 3 percent. than 2 to the finished product. The fin- (d) [Reserved] ished product must have a minimum (e) Dioxybenzone up to 3 percent. SPF of not less than the number of (f) Homosalate up to 15 percent. sunscreen active ingredients used in (g) [Reserved] the combination multiplied by 2. (h) Menthyl anthranilate up to 5 per- (2) Two or more sunscreen active in- cent. gredients identified in § 352.10(b), (c), (i) Octocrylene up to 10 percent. (e), (f), (i) through (l), (o), and (q) may (j) Octyl methoxycinnamate up to 7.5 be combined with each other in a single percent. product when used in the concentra- (k) Octyl salicylate up to 5 percent. tions established for each ingredient in (l) Oxybenzone up to 6 percent. § 352.10. The concentration of each ac- (m) Padimate O up to 8 percent. tive ingredient must be sufficient to (n) Phenylbenzimidazole sulfonic contribute a minimum SPF of not less acid up to 4 percent. than 2 to the finished product. The fin- (o) Sulisobenzone up to 10 percent. ished product must have a minimum (p) Titanium dioxide up to 25 percent. SPF of not less than the number of (q) Trolamine salicylate up to 12 per- sunscreen active ingredients used in cent. the combination multiplied by 2. (r) Zinc oxide up to 25 percent. (b) Combinations of sunscreen and skin [64 FR 27687, May 21, 1999] protectant active ingredients. Any single sunscreen active ingredient or any per- EFFECTIVE DATE NOTE: At 67 FR 41823, June mitted combination of sunscreen ac- 20, 2002, § 352.10 was amended by revising tive ingredients when used in the con- paragraphs (f) through (n), effective Sept. -

Four Common Sunscreen Questions – Spring 2015
Four common sunscreen questions – Spring 2015 Use this information SPF to help you advise 30 your customers about Use the Sun Protection System sunscreen use. 30 SP F 1. How do sunscreens filter the slip, slop, slap, and wrap sun’s rays? Sunscreens may contain physical or chemical Ethoxyethyl p-methoxycinnamate barriers to screen skin against the sun’s (cinoxate)) ultraviolet radiation (UV radiation). While physical barriers reflect or scatter the UV rays, » Salicylates (Homomenthyl salicylate chemical barriers act by absorbing the UV (homosalate), Ethylhexyl salicylate (octyl radiation before it hits the skin. salicylate/octisalate), Trolamine salicylate General advice is to choose a sunscreen which » Avobenzone (butyl methoxydibenzoylmethane) is broad spectrum, which means it filters both UVA and UVB rays. Those who have sensitive » Ecamsule ((terephthalylidene dicamphor skin should choose a sunscreen labelled sulfonic acid; Mexoryl SX) hypoallergenic or low irritant. » Ensulizole ((phenylbenzimidazole sulfonic Typical chemical absorbers include: acid) » Aminobenzoic acid derivatives (Glyceryl » Bemotrizinol (Tinosorb S) PABA), Padimate O, Roxadimate) » Bisoctrizole (Tinosorb M) » (Dioxybenzone, Benzophenones Physical blockers are titanium dioxide and Oxybenzone, Sulisonbenzone) zinc oxide. When applied they leave a thick » Cinnamates (Octocrylene, Octyl opaque (white) layer on the skin. Microfine or methoxycinnamate (octinoxate), nanoparticles of both are transparent on the skin and reflect UV radiation. 2. Do babies’ need a special sunscreen? » Babies’ skin is very sensitive and can sunburn easily so keep them out of the sun. » Once a baby is moving use the sun protection system. » Patch test an untried sunscreen on the skin 24 hours before applying it widely to check that there is no reaction. -

Lack of UV-A Protection in Daily Moisturizing Creams
rity of the data and the accuracy of the data analysis. Study concept and design: Parker and Kirsner. Acquisition of data: Parker and Kirsner. Analysis and interpretation of data: Yin, Atlantic Ocean Parker, Hu, and Kirsner. Drafting of the manuscript: Yin and Parker. Critical revision of the manuscript for important in- tellectual content: Hu and Kirsner. Obtained funding: Kirsner. N Administrative, technical, and material support: Parker and W E Kirsner. Study supervision: Kirsner. S Financial Disclosure: None reported. Funding/Support: This study was supported by the Cen- ters for Disease Control and Prevention. Role of the Sponsors: The sponsors had no role in the design and conduct of the study; in the collection, analy- sis, and interpretation of data; or in the preparation, re- view, or approval of the manuscript. Hispanic population, % 9.5-35.2 1. Rouhani P, Hu S, Kirsner RS. Melanoma in Hispanic and black Americans. 35.3-50.4 Cancer Control. 2008;15(3):248-253. 2. Kirsner RS. Cancer among indigent populations in Miami-Dade County: fi- 50.5-69.2 nal report to the American Cancer Society, Florida Division, Inc. http://sfccc 69.3-92.4 .med.miami.edu/sfccc/FCCCIDownload/MiamiDadeZipCodeReportAug05 .pdf. Accessed July 30, 2010. Late-stage diagnoses, % 3. Sylvester Comprehensive Cancer Center; Florida Department of Health. 0 Florida Cancer Data System. http://fcds.med.miami.edu/inc/idea.shtml#. Ac- 0.1-12.9 cessed July 30, 2010. 4. National Cancer Institute. SEER Summary Stage 2000: melanoma of the skin, 13.0-23.1 vulva, penis and scrotum staging. http://training.seer.cancer.gov/melanoma 23.2-44.4 /abstract-code-stage/staging.html. -

Federal Register/Vol. 84, No. 38/Tuesday, February 26, 2019
6204 Federal Register / Vol. 84, No. 38 / Tuesday, February 26, 2019 / Proposed Rules DEPARTMENT OF HEALTH AND the public. Similarly, if your submission 1978–N–0018 (formerly Docket No. HUMAN SERVICES includes safety and effectiveness data or FDA–1978–N–0038) for ‘‘Sunscreen information marked as confidential by a Drug Products for Over-the-Counter Food and Drug Administration third party (such as a contract research Human Use.’’ Received comments, those organization or consultant), you should filed in a timely manner (see 21 CFR Parts 201, 310, 347, and 352 either include a statement that you are ADDRESSES), will be placed in the docket [Docket No. FDA–1978–N–0018] (Formerly authorized to make the information and, except for those submitted as Docket No. FDA–1978–N–0038) publicly available or include an ‘‘Confidential Submissions,’’ publicly authorization from the third party viewable at https://www.regulations.gov RIN 0910–AF43 permitting the information to be or at the Dockets Management Staff between 9 a.m. and 4 p.m., Monday Sunscreen Drug Products for Over-the- publicly disclosed. If you submit data through Friday. Counter Human Use without confidential markings in response to this document and such • Confidential Submissions—To AGENCY: Food and Drug Administration, data includes studies or other submit a comment with confidential HHS. information that were previously information that you do not wish to be made publicly available, submit your ACTION: Proposed rule. submitted confidentially (e.g., as part of a new drug application), FDA intends to comments only as a written/paper SUMMARY: The Food and Drug presume that you intend to make such submission.