A Thesis Entitled Evaluating UVB and UVA Boosting Technologies For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Booklet
www.mmsus.com | 952.525.2005 COVID-19 PRODUCTS Midwest Mechanical Solutions is your partner in optimizing your building for a safe, healthy, and comfortable environment for all. Contact your MMS Representative at any time, we are here to help. Advanced HEPA Filtration Recirculation/Negative Pressure Units AF400NP 1400 cubic foot room / 280 CFM • Air Flow: Nominal 132 l/s / 280 CFM • Voltage: 120V (2A) or 230V (1A) • Dimensions: 21"H x 13"W x 16"D / 55cm x 33cm x 40cm • Filtration: 2” Pre-lter MERV10 6” Medical grade HEPA lter 99.97% (to 0.3 micron) • Optional: chemical lter • Inlet: Bottom inlet • Discharge: 4” Discharge collar • Exhaust kit included • Sound Level: Whisper quiet, 56 dBA • Controls: Remote ON/OFF, communication capabilities, audible and visual alarms AF1000NP 3000 cubic foot room / 600 CFM • Air Flow: Nominal 283 l/s / 600 CFM • Voltage: 120V (2A) or 230V (1A) • Dimensions: 56"H x 16"W x 22"D / 132cm x 40cm x 56cm • Filtration: 2” Pre-lter MERV10 12” High capacity pleated lter MERV12 6” Medical grade HEPA lter 99.97% (down to 0.3 micron) • Optional: chemical lter (up to 50 lbs./23 kg) • Inlet: Bottom inlet • Discharge: 6” Discharge collar (4”, 8” optional) • Exhaust kit included • Sound Level: Whisper quiet, 54 dBA Controls: • Remote ON/OFF, communication capabilities, audible and visual alarms AF2000NP 5000 cubic foot room / 1000 CFM • Air Flow: Nominal 283 l/s / 600 CFM • Voltage: 120V (2A) or 230V (1A) • Dimensions: 56"H x 16"W x 22"D / 132cm x 40cm x 56cm • Filtration: 2” Pre-lter MERV10 12” High capacity pleated lter -

Octinoxate, Octisalate, Avobenzone, Ensulizole, Homosalate
TONYMOLY MAGIC FOOD MANGO MILD SUN BLOCK- octinoxate, octisalate, avobenzone, ensulizole, homosalate cream TONYMOLY CO.,LTD Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies. ---------- ACTIVE INGREDIENT Active ingredients: Ethylhexyl Methoxycinnamate 6.9%, Ethylhexyl Salicylate 4.5%, Butyl Methoxydibenzoylmethane 3.5%, Phenylbenzimidazole Sulfonic Acid 3.5%, Homosalate 3.0% INACTIVE INGREDIENT Inactive ingredients: Water, Butylene Glycol, Alcohol Denat., Octocrylene, Phenethyl Benzoate, Aminomethyl Propanol, Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine, Triceteareth-4 Phosphate, Glycol Stearate, Ammonium Acryloyldimethyltaurate/VP Copolymer, Carbomer, PEG-2 Stearate, Fragrance(Parfum), Phenoxyethanol, Glyceryl Caprylate, Caprylyl Glycol, Mangifera Indica (Mango) Seed Butter, Disodium EDTA, Citrus Limon (Lemon) Fruit Extract, Musa Sapientum (Banana) Fruit Extract, Propylene Glycol, 1,2-Hexanediol, Citrus Aurantium Dulcis (Orange) Fruit Extract, Mangifera Indica (Mango) Fruit Extract, Psidium Guajava Fruit Extract, Citrus Paradisi (Grapefruit) Fruit Extract, Cocos Nucifera (Coconut) Fruit Extract, Actinidia Chinensis (Kiwi) Fruit Extract, Carica Papaya (Papaya) Fruit Extract, Ethylhexylglycerin PURPOSE Purpose: Sunscreen WARNINGS Warnings: For external use only Do not use on damaged or broken skin Stop use and ask a doctor if irritation occurs Keep out of reach of children DESCRIPTION Uses: - helps prevent sunburn - If used as directed with other sun protection measures Directions: decreases the risk of skin cancer and early skin aging caused by the sun Directions: For sunscreen use: - apply liberally 15 minutes before sun exposure - use a water resistant sunscreen if swimming or sweating - reapply at least every 2 hours - Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. -
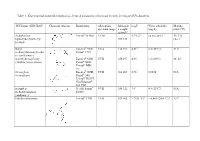
Table 1 - Experimental and Predicted Physical-Chemical Parameters of the Most Recently Investigated UV-Absorbers
Table 1 - Experimental and predicted physical-chemical parameters of the most recently investigated UV-absorbers. INCI name (INN/XAN) Chemical structure Brand name Absorption Molecula LogP Water solubility Melting spectrum range r weight (mg/L) point (°C) (g/mol)4 diethylamino Uvinul® A Plus UVA1 5.7-6.21 <0.01 (20°C) 1 54; 314 hydroxybenzoyl hexyl 397.515 (dec.) 1 benzoate Butyl Eusolex® 9020, UVA 310.393 4.514 2.2 (25°C)4 83.54 methoxydibenzoylmetha Parsol® 1789 ne (avobenzone) 4-methylbenzylidene Eusolex® 6300 UVB 258.397 4.95 1.3 (20°C) 66–68 camphor (enzacamene) Parsol® 5000 Uvinul® MBC 95 Octocrylene Eusolex® OCR, UVB 361.485 6.783 0.00383 N/A (octocrilene) Parsol® 340, Uvinul® N539T, NeoHeliopan® 303 USP isoamyl p- Neo Heliopan® UVB 248.322 3.61 4.9 (25°C)1 N/A methoxycinnamate E1000 (amiloxate) Ethylhexyl triazone Uvinul® T150 UVB 823.092 > 7(20 °C) 6 < 0.001 (20.0 °C) 6 1296 Ethylhexyl Parsol® MCX, UVB 290.403 6.14 0.041 (24 °C and N/A methoxycinnamate Heliopan® New pH 7.1) 4 (octinoxate) Ethylhexyl dimethyl Escalol™ 507 UVB 277.4084 5.774 0.54 (25 °C) 4 N/A PABA (padimate-O) Arlatone 507 Eusolex 6007 benzophenone-3 Eusolex® 4360 UVA2+ UVB 228.247 3.72 3.7 (20°C) 2 62-652 (oxybenzone) bis-ethylhexyloxyphenol Tinosorb® S UVA1+UVB 627.826 12.61 <10-4 80.401 methoxyphenol triazine (bemotrizinol) Phenylbenzimidazole Eusolex® 232 UVA2+ UVB 274.2945 -1.1 (pH 5) > 30% (As sodium N/A sulfonic acid Parsol® HS -2.1 (pH 8)5 or (ensulizole) Neo Heliopan® triethanolammoniu Hydro m salt at 20°C) 5 1 (3) 2 (34) 3 (44) 4 Pubchem 5 SCCP/1056/06 Opinion on phenylbenzimidazole sulfonic acid and its salts 6 BASF safety data sheet Table 2 – In vitro studies for the assessment of skin permeation/penetration of sunscreens. -

Sun Protection, Sunscreens and Vitamin D
SunSun protection,protection, sunscreenssunscreens andand VitaminVitamin DD GPGP NationalNational ConferenceConference RotoruaRotorua EnergyEnergy EventsEvents CentreCentre JuneJune 20092009 Dr. Louise Reiche Dermatologist New Zealand Dermatological Society Incorporated MelanomaMelanoma SkinSkin cancercancer andand sunlightsunlight Exposure to UVR causes > 90% of skin cancers Skin cancer is commonest cancer in NZ >50,000 new cases per year ~300 deaths per year ~$33.4 NZ million per year International Agency for Research on Cancer. IARC Monographs on the evaluation of carcinogenic risks to humans. Solar ultraviolet radiation. Lyon: International Agency for Research on Cancer, 1992. Armstrong BK. How sun exposure causes skin cancer. In: Hill D, Elwood JM, English DR, Eds. Prevention of Skin Cancer. Dordrecht: Kluwer Academic Publishers, 2004. O’Dea D. The Costs of Skin Cancer to New Zealand. Wellington: Cancer Society of New Zealand, 2000. New Zealand Health Information Service. Cancer, New Registrations and Deaths. Wellington: New Zealand Health Information Service, 2004. MelanomaMelanoma 1842 new cases in 2002 328 directly attributable to severe sunburn (Sneyd and Cox 2006) Authors recommended, “to reduce burden of melanoma in NZ, need to prevent excessive sun exposure and (facilitate) early diagnosis” Whilst cancer overall is rare in adolescence, melanoma was commonest cancer MelanomaMelanoma NZ incidence and death rate among world highest 56.2/100,000 in European population of Auckland highest reported worldwide men -

Sunscreen Enhancement of Octyl Methoxycinnamate Microcapsules by Using Two Biopolymers As Wall Materials
polymers Article Sunscreen Enhancement of Octyl Methoxycinnamate Microcapsules by Using Two Biopolymers as Wall Materials Chuntao Xu 1,2 , Xuemin Zeng 3, Zujin Yang 4,* and Hongbing Ji 1,3,4,5,* 1 School of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, China; [email protected] 2 School of Information Engineering, Zhongshan Polytechnic, Zhongshan 528400, China 3 Fine Chemical Industry Research Institute, School of Chemistry, Sun Yat-Sen University, Guangzhou 510275, China; [email protected] 4 School of Chemical Engineering and Technology, Sun Yat-Sen University, Zhuhai 519082, China 5 School of Chemical Engineering, Guangdong University of Petrochemical Technology, Maoming 525000, China * Correspondence: [email protected] (Z.Y.); [email protected] (H.J.) Abstract: Octyl methoxycinnamate (OMC) is widely used as a chemical sunscreen in sunscreen cosmetics. However, its direct contact with the skin would bring certain risks, such as skin photo- sensitive reaction. How to improve the effect of skin photodamage protection has become a current research hotspot. Encapsulating ultraviolet (UV) filters into microcapsules is an interesting method to increase the photostability of filters. In this study, sodium caseinate (SC) and arabic gum (GA) are chosen as wall materials to prepare synergistic sunscreen microcapsules by complex coacervation technology. A series of experiments are conducted to investigate the effects of pH, wall material concentration, and wall/core ratio on the formation of OMC microcapsules. The morphology, com- position, and stability of OMC microcapsules are characterized by scanning electron microscopy Citation: Xu, C.; Zeng, X.; Yang, Z.; Ji, (SEM), Fourier transform infrared spectroscopy (FTIR), and thermogravimetric analysis (TGA). -

FDA Proposes Sunscreen Regulation Changes February 2019
FDA Proposes Sunscreen Regulation Changes February 2019 The U.S. Food and Drug Administration (FDA) regulates sunscreens to ensure they meet safety and eectiveness standards. To improve the quality, safety, and eectiveness of sunscreens, FDA issued a proposed rule that describes updated proposed requirements for sunscreens. Given the recognized public health benets of sunscreen use, Americans should continue to use broad spectrum sunscreen with SPF 15 or higher with other sun protective measures as this important rulemaking eort moves forward. Highlights of FDA’s Proposals Sunscreen active ingredient safety and eectiveness Two ingredients (zinc oxide and titanium dioxide) are proposed to be safe and eective for sunscreen use and two (aminobenzoic acid (PABA) and trolamine salicylate) are 1 proposed as not safe and eective for sunscreen use. FDA proposes that it needs more safety information for the remaining 12 sunscreen ingredients (cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, padimate O, sulisobenzone, oxybenzone, avobenzone). New proposed sun protection factor Sunscreen dosage forms (SPF) and broad spectrum Sunscreen sprays, oils, lotions, creams, gels, butters, pastes, ointments, and sticks are requirements 2 proposed as safe and eective. FDA 3 • Raise the maximum proposed labeled SPF proposes that it needs more data for from SPF 50+ to SPF 60+ sunscreen powders. • Require any sunscreen SPF 15 or higher to be broad spectrum • Require for all broad spectrum products SPF 15 and above, as SPF increases, broad spectrum protection increases New proposed label requirements • Include alphabetical listing of active ingredients on the front panel • Require sunscreens with SPF below 15 to include “See Skin Cancer/Skin Aging alert” on the front panel 4 • Require font and placement changes to ensure SPF, broad spectrum, and water resistance statements stand out Sunscreen-insect repellent combination 5 products proposed not safe and eective www.fda.gov. -

Chemical UVR Absorbers
Chemical UVR Absorbers The names given in bold and used Diisopropyl methyl cinnamate Glyceryl ethyihexanoate dimethoxy- throughout this handbook are those of Empirical formula: cinnamate the International Nomenclature of C 6H22O2 Chemical names. Cosmetic Ingredients. Glyceryl octanoate dimethoxycinnamate; Chemical names: 2-propenoic acid, 3-(4-methoxyphenyl)-, 2-Propenoic acid, 3-12,4bis(1 diester with 1 ,3-dihydroxy-2-(2-ethyl-1 - methylethyphenyl-methyl ester; 2,5- oxohexyl)oxypropane diisopropyl methyl cinnamate _ lsoamyl-para-methoxycinnamate Ethyihexyl methoxycinnamate Empirical formula: Empirical formula: C151-12003 C 8H26O3 Chemical names: Cinnamates Chemical names: Amyl4-methoxycinnamate; isopentyl-4- 2-Ethylhexyl-4-methoxycin nam ate; methoxycinnamate; isopenlyl-para- Cinoxate 2-ethyl-hexyl-para-methoxycinnamate; methoxy-cinnamate; 3-(4-methoxyphenyl)- Empirical formula: para-methoxycinnamic acid, 2-ethylhexyl 2-propenoic acid, isopentyl ester Ci4HieO4 ester; 3-(4-methoxyphenyl)-2-propenoic acid, 2-ethylhexyl ester; octinoxate; octyl Trade names: Chemical names: methoxycinnamate; 2-propenoic acid, 3- Neo Heliopan type E 1000; Solarum AMC 2- Ethoxyothyl-para-methoxyci n nam ate; (4-methoxyphenyl)-2-ethylhexyl ester 2-propenoic acid, 3-(4-methoxyphery- para-A minobenzoic acids (PA BAs) 2-ethoxyethyl ester; 2-ethoxyethyl-4- Trade names: methoxycinnamate AEC Octyl Methoxycinnamate; Escalol Amyl dimethyl FABA 557; Eusolex 2292; Heliosol 3; Empirical formula: Trade names: Jeescreen OMC; Katoscreen OMC; Nec C14H21 NO2 Giv Tan F; Phiasol -

Les Filtres UV Dans Les Cosmétiques : Une Présence Obligatoire ?
UNIVERSITÉ DE NANTES UFR SCIENCES PHARMACEUTIQUES ET BIOLOGIQUES ____________________________________________________________________________ ANNÉE 2015 N° THÈSE pour le DIPLÔME D’ÉTAT DE DOCTEUR EN PHARMACIE par Anouk POCHAT Présentée et soutenue publiquement le 14 décembre 2015 Les filtres UV dans les cosmétiques : une présence obligatoire ? Président : Mme Laurence Coiffard, Professeur des universités, Laboratoire de Pharmacie industrielle et Cosmétologie Membres du jury : Directeur de thèse : Mme Céline Couteau, Maître de conférences, HDR, Laboratoire de Pharmacie industrielle et Cosmétologie Mme Françoise PEIGNE , Maitre de conférences à la retraite Page 1 Remerciements A mon président de jury, Professeur à la faculté des sciences pharmaceutiques de Nantes J’exprime mes profonds remerciements à Mme Coiffard, pour m’avoir fait l’honneur de présider mon jury de thèse. A mon directeur de thèse, Maître de conférences à la faculté de Pharmacie de Nantes Je remercie Mme Couteau pour m’avoir conseillée et guidée tout au long de mon travail. A Madame Françoise PEIGNE, Docteur en Pharmacie, Je remercie Mme Peigné d’avoir accepté d’assister à ma soutenance. A ma mère, Je te remercie de m’avoir soutenue et encouragée tout au long de mes études. A mon conjoint, Je te remercie pour ta patience, ton écoute et ton soutien. A mes frères, Je vous remercie pour vos encouragements Page 2 I.Introduction Une exposition prolongée aux UVA et aux UVB peut avoir de graves conséquences sur la santé comme, par exemple, la survenue de cancers cutanés (Aubin F., 2001). Les filtres UV permettent d’assurer une protection dans les domaines UVA et/ou UVB. On en trouve dans les produits de protection solaire que le public utilise ponctuellement lors des expositions prolongées au soleil. -

Bupha T 2018 Moutier La
AVERTISSEMENT Ce document est le fruit d'un long travail approuvé par le jury de soutenance et mis à disposition de l'ensemble de la communauté universitaire élargie. Il est soumis à la propriété intellectuelle de l'auteur. Ceci implique une obligation de citation et de référencement lors de l’utilisation de ce document. D'autre part, toute contrefaçon, plagiat, reproduction illicite encourt une poursuite pénale. Contact : [email protected] LIENS Code de la Propriété Intellectuelle. articles L 122. 4 Code de la Propriété Intellectuelle. articles L 335.2- L 335.10 http://www.cfcopies.com/V2/leg/leg_droi.php http://www.culture.gouv.fr/culture/infos-pratiques/droits/protection.htm UNIVERSITÉ DE LORRAINE 2018 _______________________________________________________________________________ FACULTÉ DE PHARMACIE THÈ SE Présentée et soutenue publiquement Le 21 septembre 2018 sur un sujet dédié à : LES SUBSTANCES À RISQUE DANS LES PRODUITS COSMÉTIQUES pour obtenir le Diplôme d'État de Docteur en Pharmacie par Laure MOUTIER Née le 1er septembre 1992 à Saverne (67) Membres du Jury Président : M. Bertrand RIHN Professeur en biochimie et biologie moléculaire, Université de Lorraine Directeur : M. Olivier JOUBERT Maître de conférences en toxicoloGie, Université de Lorraine JuGes : Mme Gaëlle CHARNAY Docteur en Pharmacie Mme Géraldine SCHATZ Docteur en Pharmacie UNIVERSITÉ DE LORRAINE FACULTÉ DE PHARMACIE Année universitaire 2017-2018 DOYEN Francine PAULUS Vice-Doyen/Directrice des études Virginie PICHON Conseil de la Pédagogie -

Annex VI, Last Update: 02/08/2021
File creation date: 03/10/2021 Annex VI, Last update: 22/09/2021 LIST OF UV FILTERS ALLOWED IN COSMETIC PRODUCTS Substance identification Conditions Wording of Reference Maximum conditions of Product Type, concentration Update date number Chemical name / INN / XAN Name of Common Ingredients Glossary CAS Number EC Number Other use and body parts in ready for use warnings preparation 2 N,N,N-Trimethyl-4-(2-oxoborn-3-ylidenemethyl CAMPHOR BENZALKONIUM 52793-97-2 258-190-8 6% 15/10/2010 ) anilinium methyl sulphate METHOSULFATE 3 Benzoic acid, 2-hydroxy-, HOMOSALATE 118-56-9 204-260-8 10% 02/08/2021 3,3,5-trimethylcyclohexyl ester / Homosalate 4 2-Hydroxy-4-methoxybenzophenone / BENZOPHENONE-3 131-57-7 205-031-5 6% Reg (EU) Not more than Contains 02/08/2021 Oxybenzone 2017/238 of 10 0,5 % to protect Benzophenone-3 February 2017- product (1) date of formulation application from September 2017 6 2-Phenylbenzimidazole-5-sulphonic acid and its PHENYLBENZIMIDAZOLE SULFONIC 27503-81-7 248-502-0 8%(as acid) 08/03/2011 potassium, sodium and triethanolamine salts / ACID Ensulizole 7 3,3'-(1,4-Phenylenedimethylene) bis TEREPHTHALYLIDENE DICAMPHOR 92761-26-7 / 410-960-6 / - 10%(as acid) 26/10/2010 (7,7-dimethyl-2-oxobicyclo-[2.2.1] SULFONIC ACID 90457-82-2 hept-1-ylmethanesulfonic acid) and its salts / Ecamsule 8 1-(4-tert-Butylphenyl)-3-(4-methoxyphenyl) BUTYL 70356-09-1 274-581-6 5% 15/10/2010 propane-1,3-dione / Avobenzone METHOXYDIBENZOYLMETHANE 9 alpha-(2-Oxoborn-3-ylidene)toluene-4-sulphoni BENZYLIDENE CAMPHOR SULFONIC 56039-58-8 - 6%(as acid) -

Should a Toxicological Risk Assessment of Ubiquitous Chemicals Be Done Using Data from Only One Source of Exposure?
Mini Review Open Acc J of Toxicol Volume 4 Issue 2 - January 2020 Copyright © All rights are reserved by Di Nardo JC DOI: 10.19080/OAJT.2020.04.555633 Should a Toxicological Risk Assessment of Ubiquitous Chemicals Be Done Using Data from Only One Source of Exposure? Di Nardo JC1* and CA Downs2 1Retired Toxicologist, Vesuvius, USA 2Executive Director, Haereticus Environmental Laboratory, Clifford, USA Submission: January 09, 2020; Published: January 23, 2020 *Corresponding author: Di Nardo JC, Retired Toxicologist, Vesuvius, USA Keywords: Toxicological; Chemicals; Dioxybenzone; Octocrylene; Oxybenzone; Sulisobenzone Introduction consumers to believe that there is no further risk associated with the After much discussion and petitioning by several individuals, chemical. non-governmental organizations and scientists alike, the Food & Drug Administration (FDA) re-opened the sunscreen drug monograph Data on February 26, 2019 to review the safety of sunscreen actives In October of 2018 the Food & Drug Administration (FDA) banned considered Generally Recognized as Safe and Effective (GRASE) the chemical benzophenone from use in foods and/or in plastic food since 1978 [1]. After their review, the FDA concluded that the public wraps [3], because it was found to cause cancer in rodents according to a study conducted by the National Institute of Environmental safety of a dozen drug actives currently in use (several of which record “does not” currently contain sufficient data to support the Health Sciences in May 2007 [3]. In June 2012 the state of California are benzophenone based chemicals - avobenzone, dioxybenzone, added benzophenone to their Proposition 65 list recognizing it as octocrylene, oxybenzone and sulisobenzone) and, therefore, are a carcinogen and came to an agreement that a sunscreen product requesting industry to provide additional data (mainly toxicokinetics, should not contain any more that 50 parts per million [4]. -

Jan 19, 2009 Listing of Generic, Non
http://www.medword.com/uspa.html Jan 19, 2009 Listing of generic, non-prescription, prescription, and OTC (over-the-counter) p harmaceuticals A-200 Gel Concentrate A-200 Shampoo Concentrate A-25 A-Cillin A-Fil A-Hydrocort A-methaPred A-Phedrin A-Spas S/L A Plus A.C. & C. A.P.L. A.R.M. Allergy Relief A.R.M. Maximum Strength Caplets A/B Otic A/Fish Oil A/T/S abacavir abarelix-depot-F abarelix-depot-M Abbokinase Abbokinase Open-Cath Abelcet Abenol Abitrate Absorbine Athletes Foot Absorbine Jock Itch Absorbine Jr. Antifungal AC acarbose Accolate Accupep HPF Accupril Accuretic Accutane Accutane Roche acebutolol Acel-Imune Acellular DTP Aceon Acet-2 Acet-3 Acet Codeine 30 Acet Codeine 60 Aceta Aceta Elixir Aceta Tablets acetaminophen acetaminophen-butalbital acetaminophen-caffeine acetaminophen-chlorpheniramine acetaminophen-codeine acetaminophen-dextromethorphan acetaminophen-diphenhydramine acetaminophen-hydrocodone acetaminophen-oxycodone acetaminophen-phenyltoloxamine acetaminophen-propoxyphene acetaminophen-propoxyphene hydrochloride acetaminophen-propoxyphene napsylate acetaminophen-pseudoephedrine acetazolam acetazolamide Acetest acetic acid Acetocot acetohexamide acetophenazine Acetoxyl 10 Gel Acetoxyl 2.5 Gel Acetoxyl 20 Gel Acetoxyl 5 Gel acetylsalicylic acid Achromycin Achromycin V aciclovir Acid Control Acid Phos Fluor Rinse Acilac Aciphex acitretin Aclophen Aclovate Acne-10 Lotion Acne-5 Lotion Acne-Aid Aqua Gel Acne-Aid Gel Acne-Aid Vanishing Cream Acne Aid 10 Cream Acne Lotion 10 Acne Prone Skin Sunscreen Acne Wash Acno Acnomel