Cutaneous Permeation and Penetration of Sunscreens: Formulation Strategies and in Vitro Methods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety of Oxybenzone: Putting Numbers Table
Safety of Oxybenzone: Putting Numbers Table. Years of Daily Sunscreen Application Required by an Average US Woman to Reach Systemic Levels Into Perspective of Oxybenzone per Unit of Body Mass Equivalent to Those Given to Immature Rats10 xybenzone, an organic UV-B and short-wave UV-A filter, has been available for over 40 years1; Scenario Scenario Scenario it is widely used in sunscreens and other con- Characteristic 1 2 3 O 2 sumer products in the United States. The Centers for Dis- Body surface area covered, % 100 100 25 ease Control and Prevention has estimated the preva- Application dose, mg/cm2/d211 lence of oxybenzone exposure in the general US Application amount required, 30.00 15.00 3.75 population to be 96.8%.3 In the past few years, oxyben- mL/d Time required, y 34.6 69.3 277.0 zone has received increasing attention as a potentially harmful compound. Initial concerns arose when a re- port demonstrated systemic absorption of oxybenzone in humans at a rate of 1% to 2% after topical applica- For the purposes of this estimation, a generous in-use dose of 1 mg/cm2 was used,13-16 and it was assumed that cov- tion.4 Similar or higher rates of cutaneous absorption in 5-9 erage of 25% of the human BSA was limited to the face, human subjects have been observed. The potential for neck, hands, and arms.17 biological effects, however, were first published in a study 10 by Schlumpf et al demonstrating uterotropic effects in Results. The numbers of years of daily application re- immature rats after oral administration of oxybenzone; quired to obtain the equivalent amount of sunscreen un- it should be noted that the estrogenic effect detected was der the 3 scenarios are listed in the Table. -

Download Booklet
www.mmsus.com | 952.525.2005 COVID-19 PRODUCTS Midwest Mechanical Solutions is your partner in optimizing your building for a safe, healthy, and comfortable environment for all. Contact your MMS Representative at any time, we are here to help. Advanced HEPA Filtration Recirculation/Negative Pressure Units AF400NP 1400 cubic foot room / 280 CFM • Air Flow: Nominal 132 l/s / 280 CFM • Voltage: 120V (2A) or 230V (1A) • Dimensions: 21"H x 13"W x 16"D / 55cm x 33cm x 40cm • Filtration: 2” Pre-lter MERV10 6” Medical grade HEPA lter 99.97% (to 0.3 micron) • Optional: chemical lter • Inlet: Bottom inlet • Discharge: 4” Discharge collar • Exhaust kit included • Sound Level: Whisper quiet, 56 dBA • Controls: Remote ON/OFF, communication capabilities, audible and visual alarms AF1000NP 3000 cubic foot room / 600 CFM • Air Flow: Nominal 283 l/s / 600 CFM • Voltage: 120V (2A) or 230V (1A) • Dimensions: 56"H x 16"W x 22"D / 132cm x 40cm x 56cm • Filtration: 2” Pre-lter MERV10 12” High capacity pleated lter MERV12 6” Medical grade HEPA lter 99.97% (down to 0.3 micron) • Optional: chemical lter (up to 50 lbs./23 kg) • Inlet: Bottom inlet • Discharge: 6” Discharge collar (4”, 8” optional) • Exhaust kit included • Sound Level: Whisper quiet, 54 dBA Controls: • Remote ON/OFF, communication capabilities, audible and visual alarms AF2000NP 5000 cubic foot room / 1000 CFM • Air Flow: Nominal 283 l/s / 600 CFM • Voltage: 120V (2A) or 230V (1A) • Dimensions: 56"H x 16"W x 22"D / 132cm x 40cm x 56cm • Filtration: 2” Pre-lter MERV10 12” High capacity pleated lter -

A Thesis Entitled Evaluating UVB and UVA Boosting Technologies For
A Thesis entitled Evaluating UVB and UVA Boosting Technologies for Chemical and Physical Sunscreens by An Ngoc Hiep Huynh Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science Degree in Pharmaceutical Sciences Industrial Pharmacy ___________________________________________ Gabriella Baki, Ph.D., Committee Chair ___________________________________________ Jerry Nesamony, Ph.D., Committee Member ___________________________________________ Matthew W. Liberatore, Ph.D., Committee Member ___________________________________________ Dr. Amanda C. Bryant-Friedrich, Dean College of Graduate Studies The University of Toledo May 2020 Copyright 2020 An Ngoc Hiep Huynh This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Evaluating UVB and UVA Boosting Technologies for Chemical and Physical Sunscreens by An Ngoc Hiep Huynh Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science Degree in Pharmaceutical Sciences Industrial Pharmacy The University of Toledo May 2020 There are currently 14 organic and 2 inorganic UV filters approved in the United States. Due to coral reef safety concerns, octinoxate and oxybenzone have been banned in Hawaii, Key West, FL and the US Virgin Islands; and octocrylene is also being studied for its potential impact on coral reef safety, leaving 11 organic UV filters as viable options for sunscreen manufacturers – with limitations on their combination. Since consumers are always looking for sunscreens with high SPF and broad-spectrum protection, the need for UVB and UVA protection boosting technologies is greater than ever. In a preliminary study, about two dozen emollients were scanned for their SPF boosting capability with selected organic UV filters. -

GAO-18-61, SUNSCREEN: FDA Reviewed Applications For
United States Government Accountability Office Report to Congressional Committees November 2017 SUNSCREEN FDA Reviewed Applications for Additional Active Ingredients and Determined More Data Needed GAO-18-61 November 2017 SUNSCREEN FDA Reviewed Applications for Additional Active Ingredients and Determined More Data Needed Highlights of GAO-18-61, a report to congressional committees Why GAO Did This Study What GAO Found Using sunscreen as directed with other The Food and Drug Administration (FDA), within the Department of Health and sun protective measures may help Human Services, implemented requirements for reviewing applications for reduce the risk of skin cancer—the sunscreen active ingredients within time frames set by the Sunscreen Innovation most common form of cancer in the Act, which was enacted in November 2014. For example, the agency issued a United States. In the United States, guidance document on safety and effectiveness testing in November 2016. sunscreen is considered an over-the- counter drug, which is a drug available As of August 2017, all applications for sunscreen active ingredients remain to consumers without a prescription. pending after the agency determined more safety and effectiveness data are Some sunscreen active ingredients not needed. By February 2015, FDA completed its initial review of the safety and currently marketed in the United States effectiveness data for each of the eight pending applications, as required by the have been available in products in act. FDA concluded that additional data are needed to determine that the other countries for more than a ingredients are generally recognized as safe and effective (GRASE), which is decade. Companies that manufacture needed so that products using the ingredients can subsequently be marketed in some of these ingredients have sought the United States without FDA’s premarket approval. -

Research Article Development and Validation of a Stability Indicating
Hindawi Publishing Corporation ISRN Chromatography Volume 2013, Article ID 506923, 12 pages http://dx.doi.org/10.1155/2013/506923 Research Article Development and Validation of a Stability Indicating RP-HPLC Method for the Determination of Two Sun Protection Factors (Koptrizon and Tinosorb S) in Topical Pharmaceutical Formulations Using Experimental Designs Chinmoy Roy1,2 and Jitamanyu Chakrabarty2 1 Analytical Research and Development, Dr. Reddy’s Laboratories Ltd., Bachupally, Hyderabad, Andhra Pradesh 500090, India 2 Department of Chemistry, National Institute of Technology, Durgapur, West Bengal 713209, India Correspondence should be addressed to Chinmoy Roy; [email protected] Received 11 March 2013; Accepted 15 April 2013 Academic Editors: C. Akbay and J. A. P. Coelho Copyright © 2013 C. Roy and J. Chakrabarty. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. A novel, simple, validated stability indicating HPLC method was developed for determination of Koptrizon and Tinosorb S. Stability indicating power of the method was established by forced degradation study. The chromatographic separation was achieved with Waters X Bridge C18 column, by using mobile phase consisting of a mixture of acetonitrile : tetrahydrofuran : water (38 : 38 : 24, v/v/v). The method fulfilled validation criteria and was shown to be sensitive, with limits of detection (LOD) and quantitation −1 −1 (LOQ) of 0.024 and 0.08 gmL for Koptrizon and 0.048 and 0.16 gmL for Tinosorb S, respectively. The developed method is validated for parameters like precision, accuracy, linearity, solution stability, specificity, and ruggedness as per ICH norms. -

Sun Lotion Chemicals As Endocrine Disruptors
HORMONES 2015, 14(1):32-46 Review Sun lotion chemicals as endocrine disruptors Sotirios Maipas, Polyxeni Nicolopoulou-Stamati National and Kapodistrian University of Athens, School of Medicine, First Department of Pathology and Cytology Unit, 1st Pathology Laboratory, Athens, Greece Both authors contributed equally to this work ABSTRACT Ultraviolet solar radiation is a well-known environmental health risk factor and the use of sun lotions is encouraged to achieve protection mainly from skin cancer. Sun lotions are cosmetic commercial products that combine active and inactive ingredients and many of these are associated with health problems, including allergic reactions and endocrine disorders. This review focuses on their ability to cause endocrine and reproductive impairments, with empha- sis laid on the active ingredients (common and less common UV filters). In vitro and in vivo studies have demonstrated their ability to show oestrogenic/anti-oestrogenic and androgenic/ anti-androgenic activity. Many ingredients affect the oestrous cycle, spermatogenesis, sexual behaviour, fertility and other reproductive parameters in experimental animals. Their presence in aquatic environments may reveal a new emerging environmental hazard. Key words: Active ingredients, Endocrine disruptors, Environmental hazard, Reproductive impair- ments, Sun creams, Sun lotions, Sunscreens, UV filters 1. INTRODUCTION ing, but the level of photoprotection is insufficient to prevent the harmful effects of UV radiation.3,4 Ultraviolet (UV) solar radiation is one -
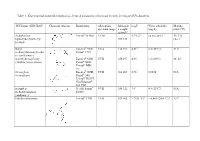
Table 1 - Experimental and Predicted Physical-Chemical Parameters of the Most Recently Investigated UV-Absorbers
Table 1 - Experimental and predicted physical-chemical parameters of the most recently investigated UV-absorbers. INCI name (INN/XAN) Chemical structure Brand name Absorption Molecula LogP Water solubility Melting spectrum range r weight (mg/L) point (°C) (g/mol)4 diethylamino Uvinul® A Plus UVA1 5.7-6.21 <0.01 (20°C) 1 54; 314 hydroxybenzoyl hexyl 397.515 (dec.) 1 benzoate Butyl Eusolex® 9020, UVA 310.393 4.514 2.2 (25°C)4 83.54 methoxydibenzoylmetha Parsol® 1789 ne (avobenzone) 4-methylbenzylidene Eusolex® 6300 UVB 258.397 4.95 1.3 (20°C) 66–68 camphor (enzacamene) Parsol® 5000 Uvinul® MBC 95 Octocrylene Eusolex® OCR, UVB 361.485 6.783 0.00383 N/A (octocrilene) Parsol® 340, Uvinul® N539T, NeoHeliopan® 303 USP isoamyl p- Neo Heliopan® UVB 248.322 3.61 4.9 (25°C)1 N/A methoxycinnamate E1000 (amiloxate) Ethylhexyl triazone Uvinul® T150 UVB 823.092 > 7(20 °C) 6 < 0.001 (20.0 °C) 6 1296 Ethylhexyl Parsol® MCX, UVB 290.403 6.14 0.041 (24 °C and N/A methoxycinnamate Heliopan® New pH 7.1) 4 (octinoxate) Ethylhexyl dimethyl Escalol™ 507 UVB 277.4084 5.774 0.54 (25 °C) 4 N/A PABA (padimate-O) Arlatone 507 Eusolex 6007 benzophenone-3 Eusolex® 4360 UVA2+ UVB 228.247 3.72 3.7 (20°C) 2 62-652 (oxybenzone) bis-ethylhexyloxyphenol Tinosorb® S UVA1+UVB 627.826 12.61 <10-4 80.401 methoxyphenol triazine (bemotrizinol) Phenylbenzimidazole Eusolex® 232 UVA2+ UVB 274.2945 -1.1 (pH 5) > 30% (As sodium N/A sulfonic acid Parsol® HS -2.1 (pH 8)5 or (ensulizole) Neo Heliopan® triethanolammoniu Hydro m salt at 20°C) 5 1 (3) 2 (34) 3 (44) 4 Pubchem 5 SCCP/1056/06 Opinion on phenylbenzimidazole sulfonic acid and its salts 6 BASF safety data sheet Table 2 – In vitro studies for the assessment of skin permeation/penetration of sunscreens. -

Two-Step Semi-Microscale Preparation of a Cinnamate Ester Sunscreen Analog W
In the Laboratory edited by The Microscale Laboratory R. David Crouch Dickinson College Carlisle, PA 17013-2896 Two-Step Semi-Microscale Preparation of a Cinnamate Ester Sunscreen Analog W Ryan G. Stabile and Andrew P. Dicks* Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario, Canada, M5S 3H6; *[email protected] Several laboratory experiments concerning the physical A two-step procedure using 4-methoxybenzaldehyde as properties of sunscreens have appeared in this Journal dur- the starting material (Scheme I) synthesizes ethyl trans-4- ing the last decade (1–5). These include quantification of methoxycinnamate 1. In the first laboratory session students commercial formulations by liquid chromatography (1) and are exposed to an important carbon–carbon bond forming ultraviolet (UV) spectrophotometry (2–4). The photochem- condensation reaction. The Verley–Doebner modification of istry of sunscreens has also been reviewed (6). Significantly, the Knoevenagel condensation (11) affords facile synthesis a student procedure focusing on multistep sunscreen synthesis of trans-4-methoxycinnamic acid, which is isolated and char- and spectroscopic analysis has not, to our knowledge, been acterized. During the second period, esterification is effected reported. Given the current high profile nature of skin can- by a cesium base mediated O-alkylation approach. This ex- cer (7) and media attention towards sunscreens, we designed emplifies the so-called “cesium effect” (12) and the useful- a two-step synthetic pathway towards an analog of a com- ness of cesium carboxylates as nucleophiles in SN2 reactions. mercially available UV light blocker. This methodology is in- In addition to stimulating class enthusiasm towards syn- corporated into a third-year undergraduate organic synthesis thetic chemistry, this experiment impresses many organic course at the University of Toronto. -

Sunscreen Enhancement of Octyl Methoxycinnamate Microcapsules by Using Two Biopolymers As Wall Materials
polymers Article Sunscreen Enhancement of Octyl Methoxycinnamate Microcapsules by Using Two Biopolymers as Wall Materials Chuntao Xu 1,2 , Xuemin Zeng 3, Zujin Yang 4,* and Hongbing Ji 1,3,4,5,* 1 School of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, China; [email protected] 2 School of Information Engineering, Zhongshan Polytechnic, Zhongshan 528400, China 3 Fine Chemical Industry Research Institute, School of Chemistry, Sun Yat-Sen University, Guangzhou 510275, China; [email protected] 4 School of Chemical Engineering and Technology, Sun Yat-Sen University, Zhuhai 519082, China 5 School of Chemical Engineering, Guangdong University of Petrochemical Technology, Maoming 525000, China * Correspondence: [email protected] (Z.Y.); [email protected] (H.J.) Abstract: Octyl methoxycinnamate (OMC) is widely used as a chemical sunscreen in sunscreen cosmetics. However, its direct contact with the skin would bring certain risks, such as skin photo- sensitive reaction. How to improve the effect of skin photodamage protection has become a current research hotspot. Encapsulating ultraviolet (UV) filters into microcapsules is an interesting method to increase the photostability of filters. In this study, sodium caseinate (SC) and arabic gum (GA) are chosen as wall materials to prepare synergistic sunscreen microcapsules by complex coacervation technology. A series of experiments are conducted to investigate the effects of pH, wall material concentration, and wall/core ratio on the formation of OMC microcapsules. The morphology, com- position, and stability of OMC microcapsules are characterized by scanning electron microscopy Citation: Xu, C.; Zeng, X.; Yang, Z.; Ji, (SEM), Fourier transform infrared spectroscopy (FTIR), and thermogravimetric analysis (TGA). -

Photophysics and Skin Penetration of Active Agents in a Commercial Sunscreen and Insect Repellent
PHOTOPHYSICS AND SKIN PENETRATION OF ACTIVE AGENTS IN A COMMERCIAL SUNSCREEN AND INSECT REPELLENT by DONALD PRETTYPAUL A Dissertation submitted to the Graduate School-Newark Rutgers, The State University of New Jersey In partial fulfillment of the requirements for the degree of Doctor of Philosophy Graduate Program in Chemistry written under the direction of Professor Richard Mendelsohn Professor Piotr Piotrowiak and approved by Newark, New Jersey October 2018 ©2018 Donald Prettypaul ALL RIGHTS RESERVED ABSTRACT OF DISSERTATION PHOTOPHYSICS AND SKIN PENETRATION OF ACTIVE AGENTS IN A COMMERCIAL SUNSCREEN AND INSECT REPELLENT By DONALD PRETTYPAUL Dissertation co-Directors: Professor Richard Mendelsohn Professor Piotr Piotrowiak This dissertation is focused on active agents in commercial sunscreen and insect repellent products. It consists of two parts, the first focusing on the photophysics of a sunscreen active agent and the second on the permeation and spatial distribution of the sunscreen active and an insect repellent active when these agents are applied to ex-vivo human skin. In the photochemistry study, ultrafast spectroscopy was used to study the excited state dynamics of the sunscreen molecule, Bemotrizinol. The work focused on the dissipation rates of the electronic excitation energy in different solvents. To complement the results from time-resolved femtosecond spectroscopy, Hartree- Fock UH/UHF 6-31G* calculations were used to characterize the ground and excited states potential energy surfaces. The results indicate that the excited state deactivation pathway follows a proton coupled electron transfer process which ii proceeds via a concerted mechanism. The dependencies on solvent polarity, viscosity, and H/D isotope effects, were investigated. Sunscreen products have been developed to protect skin from ultraviolet (UV) radiation; to achieve adequate protection, the sunscreen must be evenly applied and remain on the surface of the skin. -

New Technology Provides Cosmetically Elegant Photoprotection Zoe Diana Draelos, MD; Christian Oresajo; Margarita Yatskayer; Angelike Galdi; Isabelle Hansenne
COSMETIC CONSULTATION New Technology Provides Cosmetically Elegant Photoprotection Zoe Diana Draelos, MD; Christian Oresajo; Margarita Yatskayer; Angelike Galdi; Isabelle Hansenne he primary preventable cause of photoaging is United States, as there is no official rating system for exposure to UVA radiation. This wavelength UVA photoprotection yet. T emitted by the sun is present year round in all Ecamsule absorbs UVA radiation in the range of 320 to latitudes. Currently, the majority of sun-protective prod- 360 nm, but its peak absorbance occurs at 345 nm. It is ucts provide excellent UVB protection with minimal UVA typically combined with other organic sunscreen ingredi- protection. Although UVB protection is important in ents, such as avobenzone and octocrylene. Avobenzone, order to prevent sun damage to the skin from occurring, which is a photounstable photoprotectant, becomes UVA protection is equally important. New developments photostable when combined with octocrylene. These in raw material science have led to the manufacture of ingredients yield a photostable broad-spectrum sunscreen novel ingredients able to provide unprecedented photo- combination. However, the active sunscreen agents are protection COSin the UVA spectrum. DERMonly part of the formulation. Also important in sunscreen One of the most significant developments in cutaneous is the construction of the vehicle to deliver the photo- UVA protection was the discovery of ecamsule (Figure 1), protectants in an aesthetically pleasing manner, enticing also known as Mexoryl SX. Mexoryl SX, which has patients to wear the product. Sunscreens fail to be effec- the International Nomenclature of Cosmetic Ingredients tive if they remain in the bottle. name of terephthalylidene dicamphor sulfonic acid, is In order to evaluate the efficacy and tolerability of a water soluble. -

Effect of 10 UV Filters on the Brine Shrimp Artemia Salina and the Marine Microalgae Tetraselmis Sp
bioRxiv preprint doi: https://doi.org/10.1101/2020.01.30.926451; this version posted January 31, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Effect of 10 UV filters on the brine shrimp Artemia salina and the marine microalgae Tetraselmis sp. Evane Thorel, Fanny Clergeaud, Lucie Jaugeon, Alice M. S. Rodrigues, Julie Lucas, Didier Stien, Philippe Lebaron* Sorbonne Université, CNRS USR3579, Laboratoire de Biodiversité et Biotechnologie Microbienne, LBBM, Observatoire Océanologique, 66650, Banyuls-sur-mer, France. *corresponding author : E-mail address : [email protected] Abstract The presence of pharmaceutical and personal care products’ (PPCPs) residues in the aquatic environment is an emerging issue due to their uncontrolled release, through grey water, and accumulation in the environment that may affect living organisms, ecosystems and public health. The aim of this study is to assess the toxicity of benzophenone-3 (BP-3), bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT), butyl methoxydibenzoylmethane (BM), methylene bis- benzotriazolyl tetramethylbutylphenol (MBBT), 2-Ethylhexyl salicylate (ES), diethylaminohydroxybenzoyl hexyl benzoate (DHHB), diethylhexyl butamido triazone (DBT), ethylhexyl triazone (ET), homosalate (HS), and octocrylene (OC) to marine organisms from two major trophic levels including autotrophs (Tetraselmis sp.) and heterotrophs (Artemia salina). In general, EC50 results show that both HS and OC are the most toxic for our tested species, followed by a significant effect of BM on Artemia salina but only at high concentrations (1 mg/L) and then an effect of ES, BP3 and DHHB on the metabolic activity of the microalgae at 100 µg/L.