Sun Lotion Chemicals As Endocrine Disruptors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GAO-18-61, SUNSCREEN: FDA Reviewed Applications For
United States Government Accountability Office Report to Congressional Committees November 2017 SUNSCREEN FDA Reviewed Applications for Additional Active Ingredients and Determined More Data Needed GAO-18-61 November 2017 SUNSCREEN FDA Reviewed Applications for Additional Active Ingredients and Determined More Data Needed Highlights of GAO-18-61, a report to congressional committees Why GAO Did This Study What GAO Found Using sunscreen as directed with other The Food and Drug Administration (FDA), within the Department of Health and sun protective measures may help Human Services, implemented requirements for reviewing applications for reduce the risk of skin cancer—the sunscreen active ingredients within time frames set by the Sunscreen Innovation most common form of cancer in the Act, which was enacted in November 2014. For example, the agency issued a United States. In the United States, guidance document on safety and effectiveness testing in November 2016. sunscreen is considered an over-the- counter drug, which is a drug available As of August 2017, all applications for sunscreen active ingredients remain to consumers without a prescription. pending after the agency determined more safety and effectiveness data are Some sunscreen active ingredients not needed. By February 2015, FDA completed its initial review of the safety and currently marketed in the United States effectiveness data for each of the eight pending applications, as required by the have been available in products in act. FDA concluded that additional data are needed to determine that the other countries for more than a ingredients are generally recognized as safe and effective (GRASE), which is decade. Companies that manufacture needed so that products using the ingredients can subsequently be marketed in some of these ingredients have sought the United States without FDA’s premarket approval. -
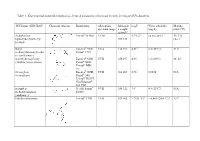
Table 1 - Experimental and Predicted Physical-Chemical Parameters of the Most Recently Investigated UV-Absorbers
Table 1 - Experimental and predicted physical-chemical parameters of the most recently investigated UV-absorbers. INCI name (INN/XAN) Chemical structure Brand name Absorption Molecula LogP Water solubility Melting spectrum range r weight (mg/L) point (°C) (g/mol)4 diethylamino Uvinul® A Plus UVA1 5.7-6.21 <0.01 (20°C) 1 54; 314 hydroxybenzoyl hexyl 397.515 (dec.) 1 benzoate Butyl Eusolex® 9020, UVA 310.393 4.514 2.2 (25°C)4 83.54 methoxydibenzoylmetha Parsol® 1789 ne (avobenzone) 4-methylbenzylidene Eusolex® 6300 UVB 258.397 4.95 1.3 (20°C) 66–68 camphor (enzacamene) Parsol® 5000 Uvinul® MBC 95 Octocrylene Eusolex® OCR, UVB 361.485 6.783 0.00383 N/A (octocrilene) Parsol® 340, Uvinul® N539T, NeoHeliopan® 303 USP isoamyl p- Neo Heliopan® UVB 248.322 3.61 4.9 (25°C)1 N/A methoxycinnamate E1000 (amiloxate) Ethylhexyl triazone Uvinul® T150 UVB 823.092 > 7(20 °C) 6 < 0.001 (20.0 °C) 6 1296 Ethylhexyl Parsol® MCX, UVB 290.403 6.14 0.041 (24 °C and N/A methoxycinnamate Heliopan® New pH 7.1) 4 (octinoxate) Ethylhexyl dimethyl Escalol™ 507 UVB 277.4084 5.774 0.54 (25 °C) 4 N/A PABA (padimate-O) Arlatone 507 Eusolex 6007 benzophenone-3 Eusolex® 4360 UVA2+ UVB 228.247 3.72 3.7 (20°C) 2 62-652 (oxybenzone) bis-ethylhexyloxyphenol Tinosorb® S UVA1+UVB 627.826 12.61 <10-4 80.401 methoxyphenol triazine (bemotrizinol) Phenylbenzimidazole Eusolex® 232 UVA2+ UVB 274.2945 -1.1 (pH 5) > 30% (As sodium N/A sulfonic acid Parsol® HS -2.1 (pH 8)5 or (ensulizole) Neo Heliopan® triethanolammoniu Hydro m salt at 20°C) 5 1 (3) 2 (34) 3 (44) 4 Pubchem 5 SCCP/1056/06 Opinion on phenylbenzimidazole sulfonic acid and its salts 6 BASF safety data sheet Table 2 – In vitro studies for the assessment of skin permeation/penetration of sunscreens. -

Sunscreen Enhancement of Octyl Methoxycinnamate Microcapsules by Using Two Biopolymers As Wall Materials
polymers Article Sunscreen Enhancement of Octyl Methoxycinnamate Microcapsules by Using Two Biopolymers as Wall Materials Chuntao Xu 1,2 , Xuemin Zeng 3, Zujin Yang 4,* and Hongbing Ji 1,3,4,5,* 1 School of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, China; [email protected] 2 School of Information Engineering, Zhongshan Polytechnic, Zhongshan 528400, China 3 Fine Chemical Industry Research Institute, School of Chemistry, Sun Yat-Sen University, Guangzhou 510275, China; [email protected] 4 School of Chemical Engineering and Technology, Sun Yat-Sen University, Zhuhai 519082, China 5 School of Chemical Engineering, Guangdong University of Petrochemical Technology, Maoming 525000, China * Correspondence: [email protected] (Z.Y.); [email protected] (H.J.) Abstract: Octyl methoxycinnamate (OMC) is widely used as a chemical sunscreen in sunscreen cosmetics. However, its direct contact with the skin would bring certain risks, such as skin photo- sensitive reaction. How to improve the effect of skin photodamage protection has become a current research hotspot. Encapsulating ultraviolet (UV) filters into microcapsules is an interesting method to increase the photostability of filters. In this study, sodium caseinate (SC) and arabic gum (GA) are chosen as wall materials to prepare synergistic sunscreen microcapsules by complex coacervation technology. A series of experiments are conducted to investigate the effects of pH, wall material concentration, and wall/core ratio on the formation of OMC microcapsules. The morphology, com- position, and stability of OMC microcapsules are characterized by scanning electron microscopy Citation: Xu, C.; Zeng, X.; Yang, Z.; Ji, (SEM), Fourier transform infrared spectroscopy (FTIR), and thermogravimetric analysis (TGA). -

Use of Vegetable Oils to Improve the Sun Protection Factor of Sunscreen Formulations
cosmetics Article Use of Vegetable Oils to Improve the Sun Protection Factor of Sunscreen Formulations Lucia Montenegro * and Ludovica Maria Santagati Department of Drug Sciences, University of Catania, Viale A. Doria 6, 95125 Catania, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-095-7384010 Received: 11 March 2019; Accepted: 1 April 2019; Published: 8 April 2019 Abstract: Some vegetable oils have many biological properties, including UV-absorbing capacity. Therefore, their use has been suggested to reduce the content of organic UV-filters in sunscreen products. In this work, we investigated the feasibility of developing oil-based vehicles with a high sun protection factor (SPF) using pomegranate oil (PMG) and shea oil (BPO) in association with different percentages of organic UV-filters (octyl– methoxycinnamate, butyl methoxydibenzoylmethane, and bemotrizinol). We characterized the spreadability, occlusion factor, pH, and required hydrophilic lipophilic balance of the resulting formulations, and did not observe relevant differences due to the incorporation of vegetable oils. The in vitro spectrophotometric determinations of SPF values highlighted that the addition of BPO (1% (w/w)) and PMG (1% (w/w)) resulted in an increase in SPF in comparison with the same formulations that contained only organic UV-filters. The SPF increase was more significant for the formulations that contained lower amounts of organic UV-filters. The results of this study supported the hypothesis that including suitable vegetable oils in sunscreen formulations could be a promising strategy to design products with a lower content of organic UV-filters. Keywords: sunscreens; vegetable oils; sun protection factor; oil-based vehicles; occlusion; spreadability 1. -

Les Filtres UV Dans Les Cosmétiques : Une Présence Obligatoire ?
UNIVERSITÉ DE NANTES UFR SCIENCES PHARMACEUTIQUES ET BIOLOGIQUES ____________________________________________________________________________ ANNÉE 2015 N° THÈSE pour le DIPLÔME D’ÉTAT DE DOCTEUR EN PHARMACIE par Anouk POCHAT Présentée et soutenue publiquement le 14 décembre 2015 Les filtres UV dans les cosmétiques : une présence obligatoire ? Président : Mme Laurence Coiffard, Professeur des universités, Laboratoire de Pharmacie industrielle et Cosmétologie Membres du jury : Directeur de thèse : Mme Céline Couteau, Maître de conférences, HDR, Laboratoire de Pharmacie industrielle et Cosmétologie Mme Françoise PEIGNE , Maitre de conférences à la retraite Page 1 Remerciements A mon président de jury, Professeur à la faculté des sciences pharmaceutiques de Nantes J’exprime mes profonds remerciements à Mme Coiffard, pour m’avoir fait l’honneur de présider mon jury de thèse. A mon directeur de thèse, Maître de conférences à la faculté de Pharmacie de Nantes Je remercie Mme Couteau pour m’avoir conseillée et guidée tout au long de mon travail. A Madame Françoise PEIGNE, Docteur en Pharmacie, Je remercie Mme Peigné d’avoir accepté d’assister à ma soutenance. A ma mère, Je te remercie de m’avoir soutenue et encouragée tout au long de mes études. A mon conjoint, Je te remercie pour ta patience, ton écoute et ton soutien. A mes frères, Je vous remercie pour vos encouragements Page 2 I.Introduction Une exposition prolongée aux UVA et aux UVB peut avoir de graves conséquences sur la santé comme, par exemple, la survenue de cancers cutanés (Aubin F., 2001). Les filtres UV permettent d’assurer une protection dans les domaines UVA et/ou UVB. On en trouve dans les produits de protection solaire que le public utilise ponctuellement lors des expositions prolongées au soleil. -

Annex VI, Last Update: 02/08/2021
File creation date: 03/10/2021 Annex VI, Last update: 22/09/2021 LIST OF UV FILTERS ALLOWED IN COSMETIC PRODUCTS Substance identification Conditions Wording of Reference Maximum conditions of Product Type, concentration Update date number Chemical name / INN / XAN Name of Common Ingredients Glossary CAS Number EC Number Other use and body parts in ready for use warnings preparation 2 N,N,N-Trimethyl-4-(2-oxoborn-3-ylidenemethyl CAMPHOR BENZALKONIUM 52793-97-2 258-190-8 6% 15/10/2010 ) anilinium methyl sulphate METHOSULFATE 3 Benzoic acid, 2-hydroxy-, HOMOSALATE 118-56-9 204-260-8 10% 02/08/2021 3,3,5-trimethylcyclohexyl ester / Homosalate 4 2-Hydroxy-4-methoxybenzophenone / BENZOPHENONE-3 131-57-7 205-031-5 6% Reg (EU) Not more than Contains 02/08/2021 Oxybenzone 2017/238 of 10 0,5 % to protect Benzophenone-3 February 2017- product (1) date of formulation application from September 2017 6 2-Phenylbenzimidazole-5-sulphonic acid and its PHENYLBENZIMIDAZOLE SULFONIC 27503-81-7 248-502-0 8%(as acid) 08/03/2011 potassium, sodium and triethanolamine salts / ACID Ensulizole 7 3,3'-(1,4-Phenylenedimethylene) bis TEREPHTHALYLIDENE DICAMPHOR 92761-26-7 / 410-960-6 / - 10%(as acid) 26/10/2010 (7,7-dimethyl-2-oxobicyclo-[2.2.1] SULFONIC ACID 90457-82-2 hept-1-ylmethanesulfonic acid) and its salts / Ecamsule 8 1-(4-tert-Butylphenyl)-3-(4-methoxyphenyl) BUTYL 70356-09-1 274-581-6 5% 15/10/2010 propane-1,3-dione / Avobenzone METHOXYDIBENZOYLMETHANE 9 alpha-(2-Oxoborn-3-ylidene)toluene-4-sulphoni BENZYLIDENE CAMPHOR SULFONIC 56039-58-8 - 6%(as acid) -

Salicylate UV-Filters in Sunscreen Formulations Compromise the Preservative System Efficacy Against Pseudomonas Aeruginosa and Burkholderia Cepacia
cosmetics Article Salicylate UV-Filters in Sunscreen Formulations Compromise the Preservative System Efficacy against Pseudomonas aeruginosa and Burkholderia cepacia Noa Ziklo, Inbal Tzafrir, Regina Shulkin and Paul Salama * Innovation department, Sharon Laboratories Ltd., Odem St. Industrial zone Ad-Halom, Ashdod 7898800, Israel; [email protected] (N.Z.); [email protected] (I.T.); [email protected] (R.S.) * Correspondence: [email protected]; Tel.: +972-54-2166476 Received: 15 July 2020; Accepted: 1 August 2020; Published: 3 August 2020 Abstract: Contamination of personal-care products are a serious health concern and therefore, preservative solutions are necessary for the costumers’ safety. High sun protection factor (SPF) sunscreen formulations are known to be difficult to preserve, due to their high ratio of organic phase containing the UV-filters. Salicylate esters such as octyl salicylate (OS) and homosalate (HS) are among the most common UV-filters currently used in the market, and can undergo hydrolysis by esterase molecules produced by contaminant microorganisms. The hydrolysis product, salicylic acid (SA) can be assimilated by certain bacteria that contain the chorismate pathway, in which its final product is pyochelin, an iron-chelating siderophore. Here, we show that OS and HS can compromise the preservative efficacy against two pathogenic important bacteria, Pseudomonas aeruginosa and Burkholderia cepacia. Challenge tests of formulations containing the UV-filters demonstrated that only bacteria with the chorismate pathway failed to be eradicated by the preservation system. mRNA expression levels of the bacterial pchD gene, which metabolizes SA to produce pyochelin, indicate a significant increase that was in correlation with increasing concentrations of both OS and HS. -

Cutaneous Permeation and Penetration of Sunscreens: Formulation Strategies and in Vitro Methods
cosmetics Review Cutaneous Permeation and Penetration of Sunscreens: Formulation Strategies and In Vitro Methods Silvia Tampucci * ID , Susi Burgalassi ID , Patrizia Chetoni ID and Daniela Monti ID Department of Pharmacy, University of Pisa, 56126 Pisa, Italy; [email protected] (S.B.); [email protected] (P.C.); [email protected] (D.M.) * Correspondence: [email protected] Received: 1 November 2017; Accepted: 7 December 2017; Published: 25 December 2017 Abstract: Sunscreens are the most common products used for skin protection against the harmful effects of ultraviolet radiation. However, as frequent application is recommended, the use of large amount of sunscreens could reflect in possible systemic absorption and since these preparations are often applied on large skin areas, even low penetration rates can cause a significant amount of sunscreen to enter the body. An ideal sunscreen should have a high substantivity and should neither penetrate the viable epidermis, the dermis and the systemic circulation, nor in hair follicle. The research of methods to assess the degree of penetration of solar filters into the skin is nowadays even more important than in the past, due to the widespread use of nanomaterials and the new discoveries in cosmetic formulation technology. In the present paper, different in vitro studies, published in the last five years, have been reviewed, in order to focus the attention on the different methodological approaches employed to effectively assess the skin permeation and retention of sunscreens. Keywords: sunscreens; formulation; in vitro methods; cutaneous permeation; skin penetration 1. Introduction The detrimental effects of human exposure to ultraviolet (UV) radiation have been widely investigated and can be immediate, as in the case of sunburns, or long-term, causing, in most cases, the formation of oxidizing species responsible of photo-aging, immunosuppression and chronic effects such as photo carcinogenicity [1,2]. -

Determination of Sun Protection Factor (SPF) of Sunscreens by Ultraviolet Spectrophotometry
Revista Brasileira de Ciências Farmacêuticas Brazilian Journal of Pharmaceutical Sciences vol. 40, n. 3, jul./set., 2004 Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry Elizângela Abreu Dutra, Daniella Almança Gonçalves da Costa e Oliveira, Erika Rosa Maria Kedor- Hackmann*, Maria Inês Rocha Miritello Santoro Departamento de Farmácia, Faculdade de Ciências Farmacêuticas, Universidade de São Paulo Uniterms: The aim of this research was to determine the sun protection factor • Sunscreens (SPF) of sunscreens emulsions containing chemical and physical • Cosmetic products sunscreens by ultraviolet spectrophotometry. Ten different • Sun protection factor commercially available samples of sunscreen emulsions of various • UV spectrophotometry manufactures were evaluated. The SPF labeled values were in the *Correspondence: range of 8 to 30. The SPF values of the 30% of the analyzed E. R. M. Kedor-Hackmann samples are in close agreement with the labeled SPF, 30% presented Departamento de Farmácia Faculdade de Ciências Farmacêuticas – SPF values above the labeled amount and 40% presented SPF UPS values under the labeled amount. The proposed spectrophotometric Av. Prof. Lineu Prestes, 580 – Bloco 13 – Cidade Universitária method is simple and rapid for the in vitro determination of SPF 05508-900 - São Paulo – SP – Brazil values of sunscreens emulsions. E-mail: [email protected] INTRODUCTION and is responsible for the damage due to sunburn. UVA radiation reaches the deeper layers of the epidermis and The rapid growth of commercially available products dermis and provokes the premature aging of the skin. containing sunscreens indicates that even though a suntan Ultraviolet radiations have been implicated as a causative is still desired, people are conscious of the possible dangers factor of skin cancer. -

Sun Protection
DRUG NEWS Recommending the Best Sun Protection Clinical Pearls: o Recommend a “broad-spectrum” sunscreen – one that covers UVB, UVA1, and UVA2. o Recommend SPF 30-50 o Advise on non-pharmacological sun protection methods o Emphasize proper sunscreen application technique o Emphasize skin protection when taking drugs known to cause photosensitivity. Familiarize yourself with known implicated drugs by referring to appendix 2. Background 1-4 The sun emits 3 types of ultraviolet (UV) radiation: UVC (100-290 nm), UVB (290-320 nm), and UVA (320 -400 nm). UVA rays can be further divided into the shorter UVA2 rays and the longer UVA1 rays. UVC rays, the shortest rays, are completely absorbed by the ozone layer, whereas UVB rays penetrate the epidermis and UVA rays, the longest rays, penetrate into the dermis. The main consequence of UVB irradiation is sunburn, but can also include immunosuppression and skin cancer. Consequences of UVA radiation include: phototoxicity (i.e. involvement in drug-induced sun sensitivity reactions), photo-aging, immunosuppression, and skin cancer. What is SPF? 2,5,6,7 It is easy to be misled by Sun Protection Factors (SPF). SPF is assessed through a standardized test by finding the ratio of the minimal dose of solar radiation that produces perceptible erythema (i.e., minimal erythema dose) on sunscreen-protected skin compared with unprotected skin. Sunburn is caused primarily by UVB rays (and shorter UVA2 rays), and thus SPF indicates mostly UVB protection. However, UVA protection is equally important since it is responsible for photo-aging and cancer. Therefore, it is important to look for the phrase “broad spectrum” when choosing a sunscreen as broad spectrum indicates both UVB and UVA protection. -

United States Patent (19) 11 Patent Number: 6,090,369 Stewart (45) Date of Patent: Jul.18, 2000
US006090369A United States Patent (19) 11 Patent Number: 6,090,369 Stewart (45) Date of Patent: Jul.18, 2000 54 SUNSCREEN FORMULATION WITH 5,585,090 12/1996 Yoshioka et al. ......................... 424/59 AVOBENZONE AND METHOD FOR 5,587,150 12/1996 Deflandre et al. .. ... 424/59 STABILIZING SUNSCREEN FORMULATION 5,605,680 2/1997 Deflandre et al. ........................ 424/59 WHICH CONTAINS AVOBENZONE 5,663.213 9/1997 Jones et al. ............................. 523/105 5,700,452 12/1997 Deckner et al. .......................... 424/59 76 Inventor: Ernest Glading Stewart, 101 W. Club Dr., Thomasville, Ga. 31799 Primary Examiner José G. Dees 21 Appl. No.: 08/868,765 ASSistant Examiner Michael A. Williamson Attorney, Agent, or Firm-Sanford J. ASman 22 Filed: Jun. 4, 1997 (51) Int. Cl." ....................................................... A61K 7/42 57 ABSTRACT 52) U.S. Cl. .................. 424/59; 424/401; 514/680 The invention relates to a photostable Screening cosmetic 58 Field of Search ................... 424/401, 59; 514/680, composition for protecting human skin against UV rayS, 514/681, 532, 534, 844, 845, 873, . comprising, comprising a Voben Zone, octyl methoxycinnamate, and an additional constituent, R, where 56) References Cited R is Selected from the group consisting of titanium dioxide and Zinc oxide. U.S. PATENT DOCUMENTS 5,514,437 5/1996 Tanner et al. ............................. 424/63 8 Claims, No Drawings 6,090,369 1 2 SUNSCREEN FORMULATION WITH In a preferred embodiment of the invention, the Sunscreen AVOBENZONE AND METHOD FOR contains octyl-methoxycinnamate in a weight percentage of STABILIZING SUNSCREEN FORMULATION less than 7.5% by weight of the formulation. -

U.S. EPA, Pesticide Product Label, SB-20 SPF 30, 04/01/2003
30S- 2j-S if /1 IC7 003 • fIN I 7DIB Jean Killoren WPC Brands, Inc. I Repel Road Jackson, WI 53037 Subject: SB-20 SPF 30 Insect Repellent EPA Registration No. 305-45 Submission dated July 27, 2000 Application dated June 26, 2002 Application dated October 30, 2002 Dear Ms. Killoren: The proposed amendment to the registration for the product cited above under The Federal Insecticide, Fungicide And Rodenticide Act, as amended revising the label in response to the DEET RED, PR Notices 2001-1 and 2001-6 and the Agency's letter of September 27,2002 is acceptable subject to the comments listed below: I) Change the second section of the First Aid Block to read: If you suspect a reaction to this product: • Discontinue use; • Take off contaminated clothing; • Rinse skin immediately with plenty of water for 15-20 minutes; • Call a poison control center or doctor for treatment advice. 2) Add the statement, "Have the product container with you when calling a poison control center or doctor or going for treatment.". 3) Add the statement, "For additional information in case of emergency call till free(insert Telephone Number), to the First Aid block. 4) Change the signal word WARNING to CAUTION. 5) In the Precautionary Statements, change the statement, "Causes substantial but temporary eye injury., to "Causes moderate eye irritation.". • 6) Move the statements, "Do not apply over cuts, wounds, sunburned or irritated skin. Do not allow children to handle this product.", from the Precautionary Labeling section to the Directions For Use section. 7) Place the storage and disposal statements in a box and add the statement, "PESTICIDE STORAGE; store in a cool, dry place inaccessible to children." 8) In the Hazard To Hurnans And Domestic Animals section, revise the statement, "Wash hands xxx tobacco.", to read, "Wash hands xxxxx using tobacco or using the toilet.".